Astin ACAS# 151201-75-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 151201-75-1 | SDF | Download SDF |
| PubChem ID | 177917 | Appearance | Powder |
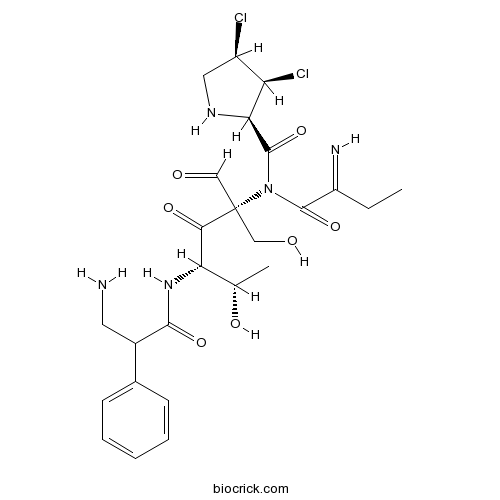
| Formula | C25H33N5O7Cl2 | M.Wt | 586.5 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3S,4R)-N-[(2S,4S,5S)-4-[(3-amino-2-phenylpropanoyl)amino]-2-formyl-1,5-dihydroxy-3-oxohexan-2-yl]-3,4-dichloro-N-(2-iminobutanoyl)pyrrolidine-2-carboxamide | ||
| SMILES | CCC(=N)C(=O)N(C(=O)C1C(C(CN1)Cl)Cl)C(CO)(C=O)C(=O)C(C(C)O)NC(=O)C(CN)C2=CC=CC=C2 | ||
| Standard InChIKey | KIIUEGNDXIENBT-DZOKUYDTSA-N | ||
| Standard InChI | InChI=1S/C25H33Cl2N5O7/c1-3-17(29)23(38)32(24(39)20-18(27)16(26)10-30-20)25(11-33,12-34)21(36)19(13(2)35)31-22(37)15(9-28)14-7-5-4-6-8-14/h4-8,11,13,15-16,18-20,29-30,34-35H,3,9-10,12,28H2,1-2H3,(H,31,37)/t13-,15?,16+,18+,19-,20-,25+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Astin A has antitumor activity. |
| Structure Identification | J Sep Sci. 2015 Feb;38(4):571-5.Simultaneous separation and determination of phenolic acids, pentapeptides, and triterpenoid saponins in the root of Aster tataricus by high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry[Pubmed: 25491750 ]
Chem Pharm Bull (Tokyo). 1996 May;44(5):1026-32.Cyclic peptides from higher plants. XXVIII. Antitumor activity and hepatic microsomal biotransformation of cyclic pentapeptides, astins, from Aster tataricus.[Pubmed: 8689717 ]
|

Astin A Dilution Calculator

Astin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.705 mL | 8.5251 mL | 17.0503 mL | 34.1006 mL | 42.6257 mL |
| 5 mM | 0.341 mL | 1.705 mL | 3.4101 mL | 6.8201 mL | 8.5251 mL |
| 10 mM | 0.1705 mL | 0.8525 mL | 1.705 mL | 3.4101 mL | 4.2626 mL |
| 50 mM | 0.0341 mL | 0.1705 mL | 0.341 mL | 0.682 mL | 0.8525 mL |
| 100 mM | 0.0171 mL | 0.0853 mL | 0.1705 mL | 0.341 mL | 0.4263 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Hydroxy-γ-sanshool
Catalog No.:BCN8849
CAS No.:78886-66-5
- Loureiriol
Catalog No.:BCN8843
CAS No.:479195-44-3
- (3R)-5,7-Dihydroxy-6-methyl-3-(4'-hydroxybenzyl)chroman-4-one
Catalog No.:BCN8842
CAS No.:84638-48-2
- Quercetin 3-O-rutinoside-1-2-O-rhamnoside
Catalog No.:BCN8820
CAS No.:55696-57-6
- Syringetin-3-O-rutinoside
Catalog No.:BCN8819
CAS No.:53430-50-5
- 6-Hydroxykaempferol 3-beta-rutinoside
Catalog No.:BCN8807
CAS No.:205527-00-0
- (+)-δ-Tocopherol
Catalog No.:BCN8805
CAS No.:119-13-1
- Silyamandin
Catalog No.:BCN8804
CAS No.:1009565-36-9
- Benzoylgomisin P
Catalog No.:BCN8803
CAS No.:129445-43-8
- Sanguisorbigenin
Catalog No.:BCN8876
CAS No.:6812-98-2
- N-trans-Sinapoyltyramine
Catalog No.:BCN8875
CAS No.:200125-11-7
- Caulophyllogenin
Catalog No.:BCN8874
CAS No.:52936-64-8
- Astin B
Catalog No.:BCN8858
CAS No.:151201-76-2
- Neochlorogenic acid methyl ester
Catalog No.:BCN8860
CAS No.:123410-65-1
- Astin C
Catalog No.:BCN8877
CAS No.:148057-23-2
- Bergaptol-beta-glucopyranoside
Catalog No.:BCN8878
CAS No.:131623-13-7
- Orthosiphol A
Catalog No.:BCN8879
CAS No.:142741-25-1
- Gypenoside XIII
Catalog No.:BCN8927
CAS No.:80325-22-0
- Tigloylgomisin O
Catalog No.:BCN8880
CAS No.:130855-74-2
- Trachelogenin 4'-O-beta-gentiobioside
Catalog No.:BCN8881
CAS No.:106647-13-6
- Geoside
Catalog No.:BCN8882
CAS No.:585-90-0
- 3-O-methylellagic acid 4'-O-alpha-L-rhamnopyranoside
Catalog No.:BCN8884
CAS No.:51768-39-9
- Pieceid-2''-O-gallate
Catalog No.:BCN8885
CAS No.:105304-51-6
- Isolappaol A
Catalog No.:BCN8886
CAS No.:131400-96-9
Antitumor astins originate from the fungal endophyte Cyanodermella asteris living within the medicinal plant Aster tataricus.[Pubmed:31811021]
Proc Natl Acad Sci U S A. 2019 Dec 6. pii: 1910527116.
Medicinal plants are a prolific source of natural products with remarkable chemical and biological properties, many of which have considerable remedial benefits. Numerous medicinal plants are suffering from wildcrafting, and thus biotechnological production processes of their natural products are urgently needed. The plant Aster tataricus is widely used in traditional Chinese medicine and contains unique active ingredients named astins. These are macrocyclic peptides showing promising antitumor activities and usually containing the highly unusual moiety 3,4-dichloroproline. The biosynthetic origins of astins are unknown despite being studied for decades. Here we show that astins are produced by the recently discovered fungal endophyte Cyanodermella asteris We were able to produce astins in reasonable and reproducible amounts using axenic cultures of the endophyte. We identified the biosynthetic gene cluster responsible for astin biosynthesis in the genome of C. asteris and propose a production pathway that is based on a nonribosomal peptide synthetase. Striking differences in the production profiles of endophyte and host plant imply a symbiotic cross-species biosynthesis pathway for astin C derivatives, in which plant enzymes or plant signals are required to trigger the synthesis of plant-exclusive variants such as Astin A. Our findings lay the foundation for the sustainable biotechnological production of astins independent from aster plants.
Simultaneous separation and determination of phenolic acids, pentapeptides, and triterpenoid saponins in the root of Aster tataricus by high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry.[Pubmed:25491750]
J Sep Sci. 2015 Feb;38(4):571-5.
We established a qualitative method to analyze the main chemical compositions of the root of Aster tataricus. Most of the peaks were separated on a C(18) column packed with 5.0 mum particles, and 28 compounds were identified, including 11 chlorogenic acids, ten astins/asterinins, and seven astersaponins, four of which were reported for the first time from A. tataricus. Furthermore, we developed a reliable method for the simultaneous quantification of 3-caffeoylquinic acid, 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, Astin A, astin B, astin C, astersaponin A, and astersaponin C, and the qualified separations were achieved only on a C18 column packed with 2.7 mum particles. The method was used to measure the concentrations of eight components in samples from two major producing areas in China, and the average contents in samples from Bozhou (Anhui) were higher than those in samples from Anguo (Hebei).


