Hydroxy-alpha-sanshoolCAS# 83883-10-7 |
- Hydroxy-beta-sanshool
Catalog No.:BCN8841
CAS No.:97465-69-5
- Hydroxy-ε-sanshool
Catalog No.:BCX1214
CAS No.:252193-26-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 83883-10-7 | SDF | Download SDF |
| PubChem ID | 10084135 | Appearance | Light Yellow Powder |
| Formula | C16H25NO2 | M.Wt | 263.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Hydroxy alpha sanshool | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
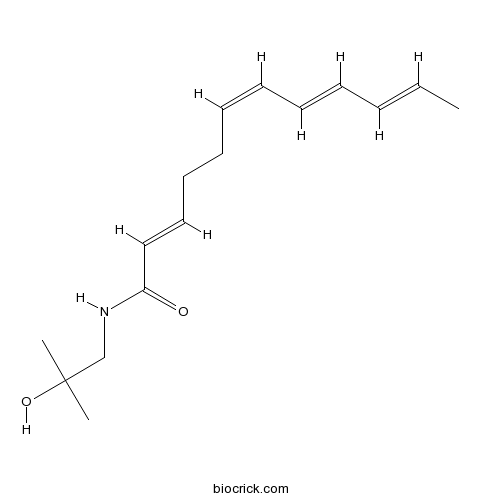
| Chemical Name | (2E,6Z,8E,10E)-N-(2-hydroxy-2-methylpropyl)dodeca-2,6,8,10-tetraenamide | ||
| SMILES | CC=CC=CC=CCCC=CC(=O)NCC(C)(C)O | ||
| Standard InChIKey | LHFKHAVGGJJQFF-UEOYEZOQSA-N | ||
| Standard InChI | InChI=1S/C16H25NO2/c1-4-5-6-7-8-9-10-11-12-13-15(18)17-14-16(2,3)19/h4-9,12-13,19H,10-11,14H2,1-3H3,(H,17,18)/b5-4+,7-6+,9-8-,13-12+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Hydroxy-alpha-sanshool exerts antiobesity and hypolipidemic activities in HFD rats by reducing liver oxidative stress and thus could be considered as a potential candidate drug to cure or prevent obesity and hyperlipidemia. It also has analgesic properties, it activates TRPV1 and TRPA1 in sensory neurons. |
| Targets | TRPV1 | TRPA1 | LDL |
| In vivo | The Plant-Derived Alkylamide, Hydroxy-Alpha-Sanshool, Induces Analgesia through Inhibition of Voltage-Gated Sodium Channels[Reference: WebLink]Biophysical Journal, 2012, 102(3):323a.Many native cultures use extracts from Xanthozylum plants to topically treat toothache and joint pain. One active component of these extracts is the alkylamide, Hydroxy-alpha-sanshool, which induces tingling and numbing paresthesia when applied to the skin or tongue. To understand the physiological mechanisms underlying paresthesias, we sought to identify the molecular targets of sanshool in the somatosensory system.
|
| Animal Research | Antiobesity, Regulation of Lipid Metabolism, and Attenuation of Liver Oxidative Stress Effects of Hydroxy-α-sanshool Isolated from Zanthoxylum bungeanum on High-Fat Diet-Induced Hyperlipidemic Rats.[Pubmed: 31534622 ]Oxid Med Cell Longev. 2019 Aug 27;2019:5852494.Zanthoxylum bungeanum is a traditional Chinese medicine (TCM) used to relieve pain, dispel dampness, stop diarrhea, and prevent itching. The aim of this study was to investigate the antiobesity and hypolipidemic effects of Hydroxy-alpha-sanshool (HAS) isolated from Z. bungeanum on hyperlipidemic rats.
|

Hydroxy-alpha-sanshool Dilution Calculator

Hydroxy-alpha-sanshool Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7965 mL | 18.9825 mL | 37.9651 mL | 75.9301 mL | 94.9127 mL |
| 5 mM | 0.7593 mL | 3.7965 mL | 7.593 mL | 15.186 mL | 18.9825 mL |
| 10 mM | 0.3797 mL | 1.8983 mL | 3.7965 mL | 7.593 mL | 9.4913 mL |
| 50 mM | 0.0759 mL | 0.3797 mL | 0.7593 mL | 1.5186 mL | 1.8983 mL |
| 100 mM | 0.038 mL | 0.1898 mL | 0.3797 mL | 0.7593 mL | 0.9491 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Quercetin 3-O-sophoroside-7-O-rhamnoside
Catalog No.:BCN8871
CAS No.:64828-40-6
- N-(p-Coumaroyl) serotonin
Catalog No.:BCN8870
CAS No.:68573-24-0
- Emodin-1-O-beta-gentiobioside
Catalog No.:BCN8869
CAS No.:849789-95-3
- Cassiaside B
Catalog No.:BCN8868
CAS No.:119170-51-3
- Piperlonguminine
Catalog No.:BCN8867
CAS No.:5950-12-9
- 7-Ethoxyrosmanol
Catalog No.:BCN8866
CAS No.:111200-01-2
- 5,7,3',4',5'-Pentamethoxyflavone
Catalog No.:BCN8865
CAS No.:53350-26-8
- Isorhamnetin 7-O-glucoside
Catalog No.:BCN8864
CAS No.:6743-96-0
- 7-Methylcoumarin
Catalog No.:BCN8863
CAS No.:2445-83-2
- Isoarnebin I
Catalog No.:BCN8862
CAS No.:24502-79-2
- Pangelin
Catalog No.:BCN8861
CAS No.:33783-80-1
- 3beta-Methoxy-2,3-dihydrowithaferin A
Catalog No.:BCN8859
CAS No.:73365-94-3
- Aegeline
Catalog No.:BCN8873
CAS No.:456-12-2
- Caulophyllogenin
Catalog No.:BCN8874
CAS No.:52936-64-8
- N-trans-Sinapoyltyramine
Catalog No.:BCN8875
CAS No.:200125-11-7
- Sanguisorbigenin
Catalog No.:BCN8876
CAS No.:6812-98-2
- Benzoylgomisin P
Catalog No.:BCN8803
CAS No.:129445-43-8
- Silyamandin
Catalog No.:BCN8804
CAS No.:1009565-36-9
- (+)-δ-Tocopherol
Catalog No.:BCN8805
CAS No.:119-13-1
- 6-Hydroxykaempferol 3-beta-rutinoside
Catalog No.:BCN8807
CAS No.:205527-00-0
- Syringetin-3-O-rutinoside
Catalog No.:BCN8819
CAS No.:53430-50-5
- Quercetin 3-O-rutinoside-1-2-O-rhamnoside
Catalog No.:BCN8820
CAS No.:55696-57-6
- (3R)-5,7-Dihydroxy-6-methyl-3-(4'-hydroxybenzyl)chroman-4-one
Catalog No.:BCN8842
CAS No.:84638-48-2
- Loureiriol
Catalog No.:BCN8843
CAS No.:479195-44-3
An Improved and Practical Method for Synthesizing of alpha-Sanshools and Spilanthol.[Pubmed:32258001]
Front Chem. 2020 Mar 17;8:187.
An efficient and practical route for the synthesis of alpha-sanshools and spilanthol is described herein. Several modifications of an existing method enabled the preparation of the (2E,6Z,8E,10E)-tetraene precursor of Hydroxy-alpha-sanshool in good yield. A highly selective Wittig reaction employing newly synthesized phosphonium salt with low deliquescence and long-term stability yielded the desired Z-form tetraene. This improved methodology was shown to be applicable to the efficient synthesis of alpha-sanshool and spilanthol.
Hydroxy-alpha-sanshool isolated from Zanthoxylum bungeanum attenuates learning and memory impairments in scopolamine-treated mice.[Pubmed:31637395]
Food Funct. 2019 Nov 1;10(11):7315-7324.
Learning and memory impairments are common symptoms of dementia in neurodegenerative disorders. Occasionally, we found that Zanthoxylum bungeanum pericarps (ZBP) significantly activated the spontaneous activity of the hippocampus (HIPP) and paraHIPP (P < 0.001, uncorrected), implying the potential ability of ZBP to improve cognitive impairments. Thus, this study aimed to investigate the improving effect of Hydroxy-alpha-sanshool (HAS), a characteristic ingredient of ZBP, against scopolamine (1 mg kg(-1), i.p.)-induced learning and memory deficits. HAS (5 mg kg(-1), p.o.) markedly reversed scopolamine-induced cognitive impairments, as indicated by its performance in the passive avoidance test and Morris water maze test (P < 0.01). Furthermore, HAS (2.5 and 5.0 mg kg(-1), p.o.) also dose-dependently prevented changes in hippocampal neuronal morphology and apoptosis, inhibited acetylcholinesterase (AChE) activity, increased the acetylcholine (ACh) content, and increased the protein and mRNA expression of brain-derived neurotrophic factor (BDNF) and phospho-cAMP response element-binding (p-CREB) compared with those in the model group (P < 0.05 & P < 0.01). These findings demonstrated that HAS attenuated scopolamine-induced cognitive impairments mainly by enhancing the activity of the cholinergic system and increasing the CREB/BDNF signalling pathway.
Simple quantitative analytical methods for the determination of alkaloids from medicinal and edible plant foods using a homemade chromatographic monolithic column.[Pubmed:31518898]
J Chromatogr B Analyt Technol Biomed Life Sci. 2019 Oct 1;1128:121784.
A polymer-based chromatographic monolithic column was prepared via in-situ radical polymerization using tetrahydrofurfuryl methacrylate as the monomer. The homemade column was used for the separation and quantitative analysis of alkaloids, including piperine from Piper longum (fruit of Piper longum Linn.) and pepper (fruit of Piper nigrum L.), Hydroxy-alpha-sanshool, and hydroxy-gamma-sanshool from zanthoxylum (fruit of Zanthoxylum bungeanum Maxim), as well as caffeine from Wuyi rock tea. The chromatographic fractions were identified by mass spectrometry. Single factor test and orthogonal test were both carried out to optimize the extraction conditions. The method validation indicated that the accuracy represented by spiked recovery ranged in 98.89%-102.06%, the correlation coefficients in 0.99986-0.99999. These results show that the prepared monolithic column can be successfully used to quantitatively analyse alkaloids from the real medicinal and edible plant foods with reversed-phase mechanism, which can avoid the long analytical time using traditional packed C18 column. The present method is a simple, and inexpensive method for quantitatively analysing alkaloids from medicinal and edible plant foods, exhibiting good specificity and durability.
Daikenchuto, a traditional Japanese herbal medicine, promotes colonic transit by inducing a propulsive movement pattern.[Pubmed:31374154]
Neurogastroenterol Motil. 2019 Nov;31(11):e13689.
BACKGROUND: The traditional Japanese herbal medicine, daikenchuto (DKT), has been used to treat constipation and postoperative ileus. However, the precise mechanisms involved in the pharmacological effects of DKT remain uncertain. The aim of this study was to clarify the effect of DKT on motor patterns and transit activity in the isolated rat colon. METHODS: The entire colon or segments of the proximal colon in rats were isolated and placed in Krebs solution. The motility of the colon was evaluated by analyzing spatiotemporal maps of diameter derived from video imaging and measuring the intraluminal pressure in the anal end of the proximal colon, and the transit time of a plastic bead through the entire isolated colon. KEY RESULTS: Several types of propagating contractions were observed in the isolated entire colon. When DKT was added to Krebs solution, the frequency of large-extent anal propagating contractions increased. DKT treatment increased the intraluminal pressure in the isolated proximal colon, which was related to the propagating contractions. This effect was abolished by treatment with the neural blocker tetrodotoxin. These findings suggest DKT induced peristaltic contractions in the isolated colon. DKT accelerated colonic transit activity, which was related to peristaltic contractions induction in the colon. These effects were also observed in the colons treated with bethanechol and the active ingredient of DKT, Hydroxy-alpha-sanshool. CONCLUSIONS AND INFERENCES: Daikenchuto could enhance colonic transit activity by inducing peristaltic contractions, which may be mediated by the activation of the enteric nervous system in the colon.
The relationship between alkylamide compound content and pungency intensity of Zanthoxylum bungeanum based on sensory evaluation and ultra-performance liquid chromatography-mass spectrometry/ mass spectrometry (UPLC-MS/MS) analysis.[Pubmed:30120773]
J Sci Food Agric. 2019 Mar 15;99(4):1475-1483.
BACKGROUND: Zanthoxylum bungeanum originating in different places varies in alkylamide content and pungency intensity. RESULTS: The pungency intensity of 19 Zanthoxylum bungeanum samples was first determined with Scoville pungency units (SPUs). The SPUs were found to range from 3.80E + 04 to 5.40E + 05. The chemical compositions and contents were measured next, using the ultra-performance liquid chromatography-mass spectrometry/ mass spectrometry (UPLC-MS/MS) method. The total alkylamide content ranged from 9.83 +/- 0.15 to 89.98 +/- 1.35 g kg(-1) . Hydroxy--sanshool, Hydroxy-alpha-sanshool, hydroxy-beta-sanshool, hydroxy-gamma-sanshool, bungeanool, and isobungeanool were found to be the key pungent compounds, ranging in proportion from 92.65% to 97.69%. The relationship between alkymide compound content and pungency intensity was also analyzed by ridge regression, and it was found that the beta values of independent variables were stable when k was more than 0.6. The regression coefficients of hydroxy--sanshool, Hydroxy-alpha-sanshool, hydroxy-beta-sanshool, hydroxy-gamma-sanshool, bungeanool, isobungeanool, and other alkylamides were 0.105, 0.177, 0.386, -0.166, -0.006, 0.005, and -0.018, respectively. CONCLUSION: Hydroxy- sanshool compounds were important in determinant the pungency intensity of Z. bungeanum. Knowledge of the relationship between alkymide compound content and pungency intensity will assist in the creation of new methods to determine pungency intensity and provide a scientific basis for flavor design, development of pungent food products, and consumer choice evaluations. (c) 2018 Society of Chemical Industry.
Dynamic Proteome Alteration and Functional Modulation of Human Saliva Induced by Dietary Chemosensory Stimuli.[Pubmed:29787679]
J Agric Food Chem. 2018 Jun 6;66(22):5621-5634.
Saliva flow measurements and SDS-PAGE separation of human whole saliva freshly collected after oral stimulation with citric acid (sour), aspartame (sweet), iso-alpha-acids (bitter), mono sodium l-glutamate (umami), NaCl (salty), 6-gingerol (pungent), Hydroxy-alpha-sanshool (tingling), and hydroxy-beta-sanshool (numbing), followed by tryptic digestion, nano-HPLC-MS/MS, and label-free protein quantitation demonstrated a stimulus- and time-dependent influence of the dietary chemosensates on salivation and the salivary proteome composition. Gene ontology enrichment analysis showed evidence for stimulus-induced alterations of the saliva proteome to boot an efficient molecular defense network of the oral cavity, e.g., 6-gingerol increased salivary lactoperoxidase activity, catalyzing the oxidation of thiocyanate to produce the antimicrobial and antifungal hypothiocyanate, from 0.37 +/- 0.02 to 0.91 +/- 0.05 mU/mL 45 s after stimulation. In comparison, oral citric acid stimulation induced an increase of myeloperoxidase activity, catalyzing the chloride oxidation to generate antimicrobial hypochloride in saliva, from 0.24 +/- 0.04 to 0.70 +/- 0.1 mU/mL as well as an increase of salivary levels of lysozyme, exhibiting antimicrobial activity on Gram-positive bacteria, from 6.0-10 to 100-150 mug/mL. Finally, microbial growth experiments clearly demonstrated for the first time that the increase of the salivary lysozyme abundance upon oral citric acid stimulation translates into an enhanced biological function, that is an almost complete growth inhibition of the two lysozyme-sensitive Gram-positive bacteria tested.
Anesthetic constituents of Zanthoxylum bungeanum Maxim.: A pharmacokinetic study.[Pubmed:27233468]
J Sep Sci. 2016 Jul;39(14):2728-35.
A sensitive and selective ultra high performance liquid chromatography with tandem mass spectrometry method was established and validated for the simultaneous determination of Hydroxy-alpha-sanshool, hydroxy-beta-sanshool, and hydroxy-gamma-sanshool in rat plasma after the subcutaneous and intravenous administration of an extract of the pericarp of Zanthoxylum bungeanum Maxim. Piperine was used as the internal standard. The analytes were extracted from rat plasma by liquid-liquid extraction with ethyl acetate and separated on a Thermo Hypersil GOLD C18 column (2.1 mm x 50 mm, 1.9 mum) with a gradient elution system at a flow rate of 0.4 mL/min. The mobile phase consisted of acetonitrile/0.05% formic acid in water and the total analysis time was 4 min. Positive electrospray ionization was performed using multiple reaction monitoring mode for the analytes. The calibration curves of the three analytes were linear over the tested concentration range. The intra- and interday precision was no more than 13.6%. Extraction recovery, matrix effect, and stability were satisfactory in rat plasma. The developed and validated method was suitable for the quantification of Hydroxy-alpha-sanshool, hydroxy-beta-sanshool, and hydroxy-gamma-sanshool and successfully applied to a pharmacokinetic study of these analytes after subcutaneous and intravenous administration to rats.
Contraction of gut smooth muscle cells assessed by fluorescence imaging.[Pubmed:25837933]
J Pharmacol Sci. 2015 Mar;127(3):344-51.
Here we discuss the development of a novel cell imaging system for the evaluation of smooth muscle cell (SMC) contraction. SMCs were isolated from the circular and longitudinal muscular layers of mouse small intestine by enzymatic digestion. SMCs were stimulated by test agents, thereafter fixed in acrolein. Actin in fixed SMCs was stained with phalloidin and cell length was determined by measuring diameter at the large end of phalloidin-stained strings within the cells. The contractile response was taken as the decrease in the average length of a population of stimulated-SMCs. Various mediators and chemically identified compounds of daikenchuto (DKT), pharmaceutical-grade traditional Japanese prokinetics, were examined. Verification of the integrity of SMC morphology by phalloidin and DAPI staining and semi-automatic measurement of cell length using an imaging analyzer was a reliable method by which to quantify the contractile response. Serotonin, substance P, prostaglandin E2 and histamine induced SMC contraction in concentration-dependent manner. Two components of DKT, Hydroxy-alpha-sanshool and hydroxy-beta-sanshool, induced contraction of SMCs. We established a novel cell imaging technique to evaluate SMC contractility. This method may facilitate investigation into SMC activity and its role in gastrointestinal motility, and may assist in the discovery of new prokinetic agents.
All-trans-configuration in Zanthoxylum alkylamides swaps the tingling with a numbing sensation and diminishes salivation.[Pubmed:24606317]
J Agric Food Chem. 2014 Mar 26;62(12):2479-88.
The methanol soluble prepared from a supercritical fluid extract of Szechuan pepper (Zanthoxylum piperitum) was screened for its key tingling and numbing chemosensates by application of taste dilution analysis. Further separation of fractions perceived with the highest sensory impact, followed by LC-TOF-MS, LC-MS, and 1D/2D NMR experiments, led to the structure determination of the known alkylamides hydroxy-gamma-sanshool (1), Hydroxy-alpha-sanshool (2), hydroxy-beta-sanshool (3), bungeanool (4), isobungeanool (5), and hydroxy-gamma-isosanshool (6), as well as hydroxy-epsilon-sanshool (7), the structure of which has not yet been confirmed by NMR, and hydroxy-zeta-sanshool (8), which has not been previously reported in the literature. Psychophysical half-tongue experiments using filter paper rectangles (1 x 2 cm) as the vehicle revealed amides 1, 2, 4, 5, 7, and 8, showing at least one cis-configured double bond, elicited the well-known tingling and paresthetic orosensation above threshold levels of 3.5-8.3 nmol/cm(2). In contrast, the all-trans-configured amides 3 and 6 induced a numbing and anesthetic sensation above thresholds of 3.9 and 7.1 nmol/cm(2), respectively. Interestingly, the mono-cis-configured major amide 2 was found to induce massive salivation, whereas the all-trans-configuration of 3 did not.


