Cassiaside BCAS# 119170-51-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 119170-51-3 | SDF | Download SDF |
| PubChem ID | 131752379 | Appearance | Powder |
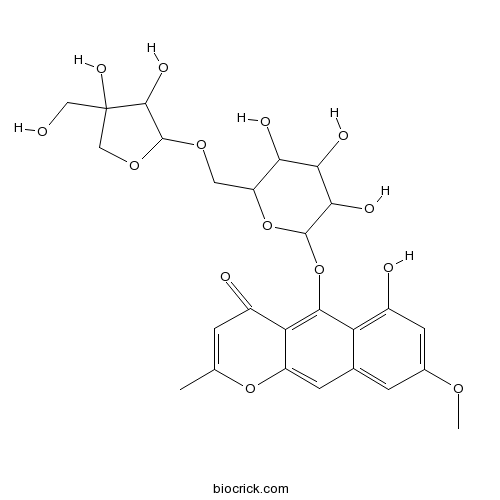
| Formula | C26H30O14 | M.Wt | 566.5 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-[6-[[3,4-dihydroxy-4-(hydroxymethyl)oxolan-2-yl]oxymethyl]-3,4,5-trihydroxyoxan-2-yl]oxy-6-hydroxy-8-methoxy-2-methylbenzo[g]chromen-4-one | ||
| SMILES | CC1=CC(=O)C2=C(C3=C(C=C(C=C3C=C2O1)OC)O)OC4C(C(C(C(O4)COC5C(C(CO5)(CO)O)O)O)O)O | ||
| Standard InChIKey | FKKPTUZRYGLMMA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C26H30O14/c1-10-3-13(28)18-15(38-10)5-11-4-12(35-2)6-14(29)17(11)22(18)40-24-21(32)20(31)19(30)16(39-24)7-36-25-23(33)26(34,8-27)9-37-25/h3-6,16,19-21,23-25,27,29-34H,7-9H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Phytochemistry. 1991;30(2):708-10.An anthraquinone and three naphthopyrone derivatives from Cassia pudibunda.[Pubmed: 1367272 ]
|

Cassiaside B Dilution Calculator

Cassiaside B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7652 mL | 8.8261 mL | 17.6523 mL | 35.3045 mL | 44.1306 mL |
| 5 mM | 0.353 mL | 1.7652 mL | 3.5305 mL | 7.0609 mL | 8.8261 mL |
| 10 mM | 0.1765 mL | 0.8826 mL | 1.7652 mL | 3.5305 mL | 4.4131 mL |
| 50 mM | 0.0353 mL | 0.1765 mL | 0.353 mL | 0.7061 mL | 0.8826 mL |
| 100 mM | 0.0177 mL | 0.0883 mL | 0.1765 mL | 0.353 mL | 0.4413 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Piperlonguminine
Catalog No.:BCN8867
CAS No.:5950-12-9
- 7-Ethoxyrosmanol
Catalog No.:BCN8866
CAS No.:111200-01-2
- 5,7,3',4',5'-Pentamethoxyflavone
Catalog No.:BCN8865
CAS No.:53350-26-8
- Isorhamnetin 7-O-glucoside
Catalog No.:BCN8864
CAS No.:6743-96-0
- 7-Methylcoumarin
Catalog No.:BCN8863
CAS No.:2445-83-2
- Isoarnebin I
Catalog No.:BCN8862
CAS No.:24502-79-2
- Pangelin
Catalog No.:BCN8861
CAS No.:33783-80-1
- 3beta-Methoxy-2,3-dihydrowithaferin A
Catalog No.:BCN8859
CAS No.:73365-94-3
- Hellebrigenin
Catalog No.:BCN8857
CAS No.:465-90-7
- Rhaponticin 6''-O-gallate
Catalog No.:BCN8856
CAS No.:94356-23-7
- Gardoside
Catalog No.:BCN8855
CAS No.:54835-76-6
- Rebaudioside E
Catalog No.:BCN8854
CAS No.:63279-14-1
- Emodin-1-O-beta-gentiobioside
Catalog No.:BCN8869
CAS No.:849789-95-3
- N-(p-Coumaroyl) serotonin
Catalog No.:BCN8870
CAS No.:68573-24-0
- Quercetin 3-O-sophoroside-7-O-rhamnoside
Catalog No.:BCN8871
CAS No.:64828-40-6
- Hydroxy-alpha-sanshool
Catalog No.:BCN8872
CAS No.:83883-10-7
- Aegeline
Catalog No.:BCN8873
CAS No.:456-12-2
- Caulophyllogenin
Catalog No.:BCN8874
CAS No.:52936-64-8
- N-trans-Sinapoyltyramine
Catalog No.:BCN8875
CAS No.:200125-11-7
- Sanguisorbigenin
Catalog No.:BCN8876
CAS No.:6812-98-2
- Benzoylgomisin P
Catalog No.:BCN8803
CAS No.:129445-43-8
- Silyamandin
Catalog No.:BCN8804
CAS No.:1009565-36-9
- (+)-δ-Tocopherol
Catalog No.:BCN8805
CAS No.:119-13-1
- 6-Hydroxykaempferol 3-beta-rutinoside
Catalog No.:BCN8807
CAS No.:205527-00-0
Rubrofusarin as a Dual Protein Tyrosine Phosphate 1B and Human Monoamine Oxidase-A Inhibitor: An in Vitro and in Silico Study.[Pubmed:31460269]
ACS Omega. 2019 Jul 3;4(7):11621-11630.
A number of nature-derived biologically active compounds comprise glycosides. In some cases, the glycosidic residue is needed for bioactivity; however, in other cases, glycosylation just improves some pharmacokinetic/dynamic parameters. The patterns of protein tyrosine phosphatase 1B (PTP1B) and human monoamine oxidase A (hMAO-A) inhibition by rubrofusarin 6-O-beta-d-glucopyranoside (1), rubrofusarin 6-O-beta-d-gentiobioside (2), rubrofusarin triglucoside (3), and Cassiaside B2 (4) were compared with the aglycone, rubrofusarin, isolated from Cassia obtusifolia seeds. Rubrofusarin showed potent inhibition against the PTP1B enzyme (IC50; 16.95 +/- 0.49 muM), and its glycosides reduced activity (IC50; 87.36 +/- 1.08 muM for 1 and >100 muM for 2-4) than did the reference drug, ursolic acid (IC50; 2.29 +/- 0.04 muM). Similarly, in hMAO-A inhibition, rubrofusarin displayed the most potent activity with an IC50 value of 5.90 +/- 0.99 muM, which was twice better than the reference drug, deprenyl HCl (IC50; 10.23 +/- 0.82 muM). An enzyme kinetic and molecular docking study revealed rubrofusarin to be a mixed-competitive inhibitor of both these enzymes. In a western blot analysis, rubrofusarin increased glucose uptake significantly and decreased the PTP1B expression in a dose-dependent manner in insulin-resistant HepG2 cells, increased the expression of phosphorylated protein kinase B (p-Akt) and phosphorylated insulin receptor substrate-1 (p-IRS1) (Tyr 895), and decreased the expression of glucose-6-phosphatase (G6Pase) and phosphoenol pyruvate carboxykinase (PEPCK), key enzymes of gluconeogenesis. Our overall results show that glycosylation retards activity; however, it reduces toxicity. Thus, Cassia seed as functional food and rubrofusarin as a base can be used for the development of therapeutic agents against comorbid diabetes and depression.
[UPLC-MS~n analysis and certification evaluation of main chemical composition in naphthopyrone reference extract of Cassiae Semen].[Pubmed:31355568]
Zhongguo Zhong Yao Za Zhi. 2019 May;44(10):2102-2109.
The main chemical constituents of naphthopyrone reference extract( NRE) with definite content and relatively fixed chemical composition were analyzed and determined. Ultra-high performance liquid chromatography-LTQ-Orbitrap XL mass spectrometry and high performance liquid chromatography were used to systematically study NRE from the aspects of main chemical components and determination. The results showed that the chemical composition of naphthopyrone reference extract of Cassiae Semen was relatively fixed,and seven naphthalopyranones were identified. Cassiaside B_2,cassiaside C_2,rubrofusarin-6-O-beta-D-gentiobioside and cassiaside C were the main chemical constituents of NRE,of which the determination and uncertainty results were( 11. 40+ 0. 26) %,( 11. 68+0. 24) %,( 16. 60+0. 22) %,( 28. 8+0. 48) %,respectively. This study contributed to the accurate evaluation of NRE and the foundation for the application of NRE in the quality control of Cassiae Semen,and provided a new idea for the replacement of single chemical reference substance by the reference extract of traditional Chinese medicine.
An anthraquinone and three naphthopyrone derivatives from Cassia pudibunda.[Pubmed:1367272]
Phytochemistry. 1991;30(2):708-10.
Chemical examination of the methanolic extract of the roots of Cassia pudibunda led to isolation of the new rubrofusarin-6-O-beta-D-glucopyranoside, quinquangulin-6-O-beta- D-apiofuranosyl-(1----6)-O-beta-D-glucopyranoside, quinquangulin-6-O-beta-D-glucopyranoside and chrysophanol dimethyl ether. Moreover the known chrysophanol, physcion, cis-3,3',5,5'-tetrahydroxy-4-methoxystilbene, trans-3,3',5,5' -tetrahydroxy-4-methoxystilbene, and Cassiaside B were identified. The antimicrobial activity of some of these compounds is also reported.


