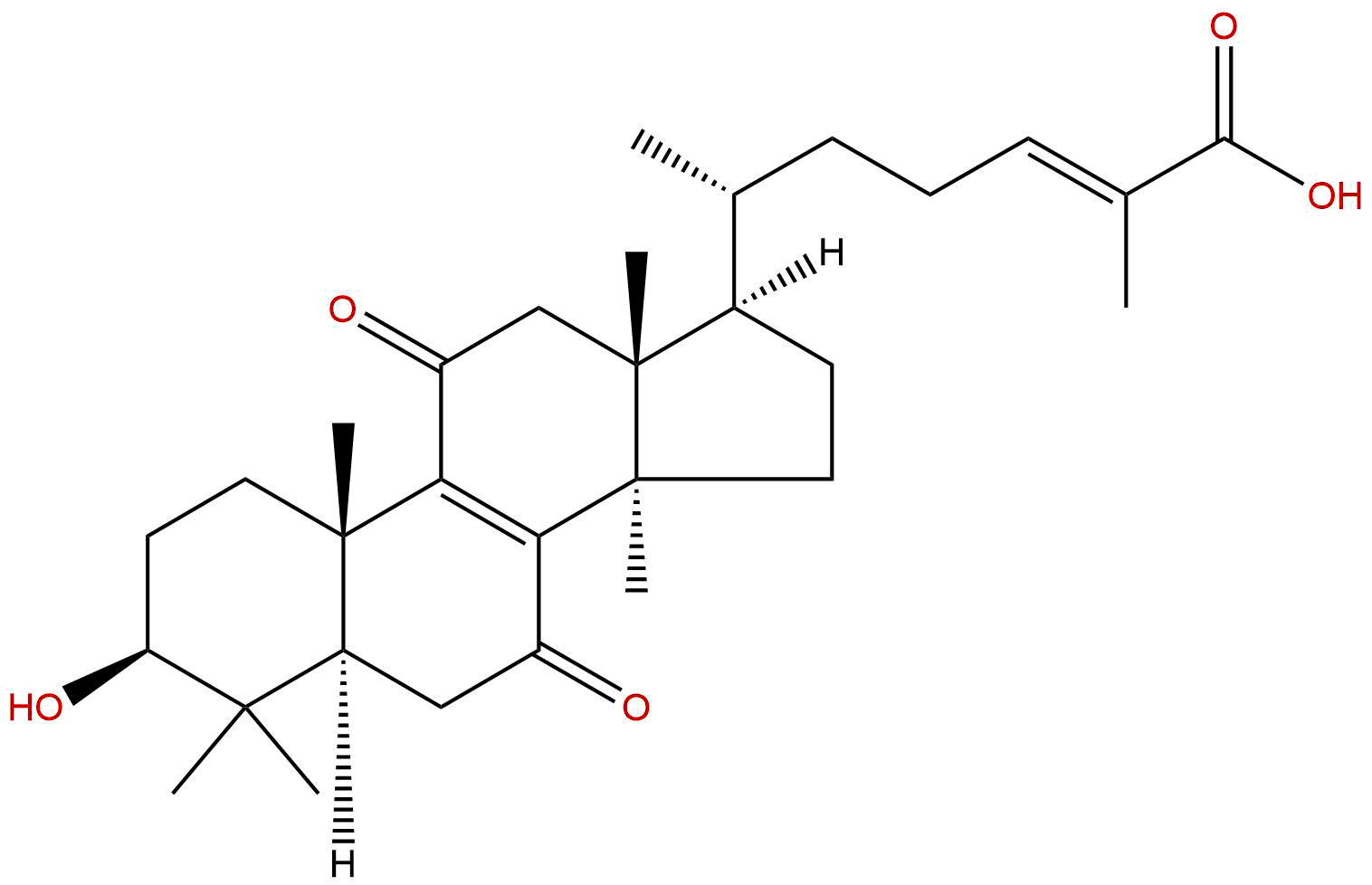
7-Oxo-ganoderic acid Z2CAS# 1446104-52-4 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 1446104-52-4 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C30H44O5 | M.Wt | 484.68 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

7-Oxo-ganoderic acid Z2 Dilution Calculator

7-Oxo-ganoderic acid Z2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0632 mL | 10.3161 mL | 20.6322 mL | 41.2643 mL | 51.5804 mL |
| 5 mM | 0.4126 mL | 2.0632 mL | 4.1264 mL | 8.2529 mL | 10.3161 mL |
| 10 mM | 0.2063 mL | 1.0316 mL | 2.0632 mL | 4.1264 mL | 5.158 mL |
| 50 mM | 0.0413 mL | 0.2063 mL | 0.4126 mL | 0.8253 mL | 1.0316 mL |
| 100 mM | 0.0206 mL | 0.1032 mL | 0.2063 mL | 0.4126 mL | 0.5158 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 15-Oxospiramilactone
Catalog No.:BCX1173
CAS No.:1053172-87-4
- Tirotundin 3-O-methyl ether
Catalog No.:BCX1172
CAS No.:1454840-36-8
- Cyaonoside B
Catalog No.:BCX1171
CAS No.:51161-58-1
- Ganolactone A
Catalog No.:BCX1170
CAS No.:173268-82-1
- Guattegaumerine
Catalog No.:BCX1169
CAS No.:21446-35-5
- 25(R)-3β,17α-Dihydroxy-5α-spirostan-6-one 3-O-α-D-rhamnopyranosyl-(1→2)-β-D-glucopyranoside
Catalog No.:BCX1168
CAS No.:143051-94-9
- Hispolon
Catalog No.:BCX1167
CAS No.:173933-40-9
- 6-methoxy-bispyranoxanthone
Catalog No.:BCX1166
CAS No.:115713-10-5
- Aucubigenin
Catalog No.:BCX1165
CAS No.:64274-28-8
- Coronafacic acid
Catalog No.:BCX1164
CAS No.:62251-98-3
- Coronatine
Catalog No.:BCX1163
CAS No.:62251-96-1
- Soyasaponin Ae
Catalog No.:BCX1162
CAS No.:117230-34-9
- Aloenin B
Catalog No.:BCX1175
CAS No.:106533-41-9
- Glucovanillin
Catalog No.:BCX1176
CAS No.:494-08-6
- Halofuginone hydrobromide
Catalog No.:BCX1177
CAS No.:64924-67-0
- 2-Hydroxypinocembrin
Catalog No.:BCX1178
CAS No.:40489-17-6
- 11-Oxo-ganoderic acid DM
Catalog No.:BCX1179
CAS No.:1408244-15-4
- GDP-L-Fuc.2Na
Catalog No.:BCX1180
CAS No.:15839-70-0
- N-Acetyl-D-lactosamine
Catalog No.:BCX1181
CAS No.:32181-59-2
- Acetylarenobufagin
Catalog No.:BCX1182
CAS No.:184673-79-8
- 5-Methoxyafrormosin 7-O-glucoside
Catalog No.:BCX1183
CAS No.:64656-92-4
- Heterophyllin J
Catalog No.:BCX1184
CAS No.:660397-15-9
- 6-Acetyl-7-hydroxy-2,3-dimethylchromone
Catalog No.:BCX1185
CAS No.:225518-70-7
- Neoisoliquiritigenin
Catalog No.:BCX1186
CAS No.:7014-39-3
[Anti-tumor target prediction and activity verification of Ganoderma lucidum triterpenoids].[Pubmed:28952258]
Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(3):517-522.
It has reported that Ganoderma lucidum triterpenoids had anti-tumor activity. However, the anti-tumor target is still unclear. The present study was designed to investigate the anti-tumor activity of G. lucidum triterpenoids on different tumor cells, and predict their potential targets by virtual screening. In this experiment, molecular docking was used to simulate the interactions of 26 triterpenoids isolated from G. lucidum and 11 target proteins by LibDock module of Discovery Studio2016 software, then the anti-tumor targets of triterpenoids were predicted. In addition, the in vitro anti-tumor effects of triterpenoids were evaluated by MTT assay by determining the inhibition of proliferation in 5 tumor cell lines. The docking results showed that the poses were greater than five, and Libdock Scores higher than 100, which can be used to determine whether compounds were activity. Eight triterpenoids might have anti-tumor activity as a result of good docking, five of which had multiple targets. MTT experiments demonstrated that the ganoderic acid Y had a certain inhibitory activity on lung cancer cell H460, with IC(5)(0) of 22.4 mumol*L (-)(1), followed by 7-Oxo-ganoderic acid Z2, with IC(5)(0) of 43.1 mumol*L (-)(1). However, the other triterpenoids had no anti-tumor activity in the detected tumor cell lines. Taking together, molecular docking approach established here can be used for preliminary screening of anti-tumor activity of G.lucidum ingredients. Through this screening method, combined with the MTT assay, we can conclude that ganoderic acid Y had antitumor activity, especially anti-lung cancer, and 7-Oxo-ganoderic acid Z2 as well as ganoderon B, to a certain extent, had anti-tumor activity. These findings can provide basis for the development of anti-tumor drugs. However, the anti-tumor mechanisms need to be further studied.
[Triterpenoids from Ganoderma theaecolum].[Pubmed:28875673]
Zhongguo Zhong Yao Za Zhi. 2016 Mar;41(6):1075-1080.
Fifteenlanostane triterpenoids were isolated from the ethanol extract of Ganoderma theaecolum by means of preparative HPLC,column chromatography over silica gel,ODS and were identified as lucidone C(1),lucidone D(2),7-Oxo-ganoderic acid Z2(3),7-oxo-ganoderic acid Z(4),ganoderenicacid H(5),ganoderenic acid B(6),3beta,7beta-dihydroyl-11,15,23-trioxo-lanost-8,16-dien-26-oic acid(7),3beta,7beta-dihydroyl-11,15,23-trioxo-lanost-8,16-dien-26-oic acid methyl ester(8),ganolucidic acid B(9),ganolucidate F(10),methyl ganoderate C2(11),ganoderic acid zeta(12),ganoderic acid AP3(13),methyl ganoderate B(14),and ganoderol B(15). Compounds 1-15 were isolated from this specie for the first time.
Protective effects of triterpenoids from Ganoderma resinaceum on H(2)O(2)-induced toxicity in HepG2 cells.[Pubmed:23790868]
Food Chem. 2013 Nov 15;141(2):920-6.
Ganoderma resinaceum Boud. (Polyporeseae) has long been used for antioxidant, immunoregulation and liver protection. From the fruiting bodies of G. resinaceum, eight new lanostanoids, lucidones D-G (1-4), 7-Oxo-ganoderic acid Z2 (5), 7-oxo-ganoderic acid Z3 (6), ganoderesin A (7), and ganoderesin B (8), together with six known lanostanoids (9-14) were isolated. The structures of new compounds were elucidated through extensive spectroscopic analysis. In an in vitro model, ganoderesin B (8), ganoderol B (10) and lucidone A (11) showed inhibitory effects against the increase of ALT and AST levels in HepG2 cells induced by H2O2 compared to a control group in the range of their maximum non-toxic concentration (MNTC). However, compounds 8, 10 and 11 displayed no anti-oxidant activities by DPPH assay. Meanwhile, activation for PXR (Pregnane X Receptor) of ganoderesin B (8), ganoderol B (10) and lucidone A (11) was evaluated; ganoderol (10) exhibited a vital activation for PXR-induced CYP3A4 expression. These results suggested that GTs (Ganoderma triterpenoids) exhibited hepatoprotective activities by lowering ALT and AST levels.


