N-Acetyl-D-lactosamineCAS# 32181-59-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 32181-59-2 | SDF | Download SDF |
| PubChem ID | 71299578.0 | Appearance | Powder |
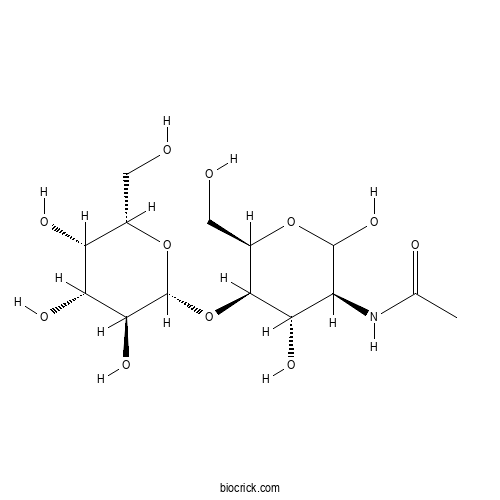
| Formula | C14H25NO11 | M.Wt | 383.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | N-[(3S,4S,5R,6R)-2,4-dihydroxy-6-(hydroxymethyl)-5-[(2R,3S,4R,5S,6S)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxan-3-yl]acetamide | ||
| SMILES | CC(=O)NC1C(C(C(OC1O)CO)OC2C(C(C(C(O2)CO)O)O)O)O | ||
| Standard InChIKey | KFEUJDWYNGMDBV-CKTWSTEBSA-N | ||
| Standard InChI | InChI=1S/C14H25NO11/c1-4(18)15-7-9(20)12(6(3-17)24-13(7)23)26-14-11(22)10(21)8(19)5(2-16)25-14/h5-14,16-17,19-23H,2-3H2,1H3,(H,15,18)/t5-,6+,7-,8+,9-,10+,11-,12-,13?,14+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

N-Acetyl-D-lactosamine Dilution Calculator

N-Acetyl-D-lactosamine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6086 mL | 13.0429 mL | 26.0858 mL | 52.1716 mL | 65.2146 mL |
| 5 mM | 0.5217 mL | 2.6086 mL | 5.2172 mL | 10.4343 mL | 13.0429 mL |
| 10 mM | 0.2609 mL | 1.3043 mL | 2.6086 mL | 5.2172 mL | 6.5215 mL |
| 50 mM | 0.0522 mL | 0.2609 mL | 0.5217 mL | 1.0434 mL | 1.3043 mL |
| 100 mM | 0.0261 mL | 0.1304 mL | 0.2609 mL | 0.5217 mL | 0.6521 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- GDP-L-Fuc.2Na
Catalog No.:BCX1180
CAS No.:15839-70-0
- 11-Oxo-ganoderic acid DM
Catalog No.:BCX1179
CAS No.:1408244-15-4
- 2-Hydroxypinocembrin
Catalog No.:BCX1178
CAS No.:40489-17-6
- Halofuginone hydrobromide
Catalog No.:BCX1177
CAS No.:64924-67-0
- Glucovanillin
Catalog No.:BCX1176
CAS No.:494-08-6
- Aloenin B
Catalog No.:BCX1175
CAS No.:106533-41-9
- 7-Oxo-ganoderic acid Z2
Catalog No.:BCX1174
CAS No.:1446104-52-4
- 15-Oxospiramilactone
Catalog No.:BCX1173
CAS No.:1053172-87-4
- Tirotundin 3-O-methyl ether
Catalog No.:BCX1172
CAS No.:1454840-36-8
- Cyaonoside B
Catalog No.:BCX1171
CAS No.:51161-58-1
- Ganolactone A
Catalog No.:BCX1170
CAS No.:173268-82-1
- Guattegaumerine
Catalog No.:BCX1169
CAS No.:21446-35-5
- Acetylarenobufagin
Catalog No.:BCX1182
CAS No.:184673-79-8
- 5-Methoxyafrormosin 7-O-glucoside
Catalog No.:BCX1183
CAS No.:64656-92-4
- Heterophyllin J
Catalog No.:BCX1184
CAS No.:660397-15-9
- 6-Acetyl-7-hydroxy-2,3-dimethylchromone
Catalog No.:BCX1185
CAS No.:225518-70-7
- Neoisoliquiritigenin
Catalog No.:BCX1186
CAS No.:7014-39-3
- Rubropunctamine
Catalog No.:BCX1187
CAS No.:514-66-9
- Glycyrrhizic acid methyl ester
Catalog No.:BCX1188
CAS No.:104191-95-9
- Kissoone A
Catalog No.:BCX1189
CAS No.:903559-01-3
- Deoxycytidine
Catalog No.:BCX1190
CAS No.:951-77-9
- Daucosterin acetate
Catalog No.:BCX1191
CAS No.:4282-00-2
- (-)-3-Demethylcolchicine
Catalog No.:BCX1192
CAS No.:7336-33-6
- Ludaconitine
Catalog No.:BCX1193
CAS No.:82144-72-7
Safety and Modulatory Effects of Humanized Galacto-Oligosaccharides on the Gut Microbiome.[Pubmed:33898497]
Front Nutr. 2021 Apr 7;8:640100.
Complex dietary carbohydrate structures including beta(1-4) galacto-oligosaccharides (GOS) are resistant to digestion in the upper gastrointestinal (GI) tract and arrive intact to the colon where they benefit the host by selectively stimulating microbial growth. Studies have reported the beneficial impact of GOS (alone or in combination with other prebiotics) by serving as metabolic substrates for modulating the assembly of the infant gut microbiome while reducing GI infections. N-Acetyl-D-lactosamine (LacNAc, Galbeta1,4GlcNAc) is found in breast milk as a free disaccharide. This compound is also found as a component of human milk oligosaccharides (HMOs), which have repeating and variably branched lactose and/or LacNAc units, often attached to sialic acid and fucose monosaccharides. Human glycosyl-hydrolases do not degrade most HMOs, indicating that these structures have evolved as natural prebiotics to drive the proper assembly of the infant healthy gut microbiota. Here, we sought to develop a novel enzymatic method for generating LacNAc-enriched GOS, which we refer to as humanized GOS (hGOS). We showed that the membrane-bound beta-hexosyl transferase (rBHT) from Hamamotoa (Sporobolomyces) singularis was able to generate GOS and hGOS from lactose and N-Acetyl-glucosamine (GlcNAc). The enzyme catalyzed the regio-selective, repeated addition of galactose from lactose to GlcNAc forming the beta-galactosyl linkage at the 4-position of the GlcNAc and at the 1-position of D-galactose generating, in addition to GOS, LacNAc, and Galactosyl-LacNAc trisaccharides which were produced by two sequential transgalactosylations. Humanized GOS is chemically distinct from HMOs, and its effects in vivo have yet to be determined. Thus, we evaluated its safety and demonstrated the prebiotic's ability to modulate the gut microbiome in 6-week-old C57BL/6J mice. Longitudinal analysis of gut microbiome composition of stool samples collected from mice fed a diet containing hGOS for 5 weeks showed a transient reduction in alpha diversity. Differences in microbiome community composition mostly within the Firmicutes phylum were observed between hGOS and GOS, compared to control-fed animals. In sum, our study demonstrated the biological synthesis of hGOS, and signaled its safety and ability to modulate the gut microbiome in vivo, promoting the growth of beneficial microorganisms, including Bifidobacterium and Akkermansia.
Galectin-3 in Prostate Cancer Stem-Like Cells Is Immunosuppressive and Drives Early Metastasis.[Pubmed:33013832]
Front Immunol. 2020 Sep 10;11:1820.
Galectin-3 (Gal-3) is an extracellular matrix glycan-binding protein with several immunosuppressive and pro-tumor functions. The role of Galectin-3 in cancer stem-like cells (CSCs) is poorly investigated. Here, we show that prostate CSCs also colonizing prostate-draining lymph nodes of transgenic adenocarcinoma of the mouse prostate (TRAMP) mice overexpress Gal-3. Gal-3 contributes to prostate CSC-mediated immune suppression because either Gal-3 silencing in CSCs, or co-culture of CSCs and T cells in the presence of the Gal-3 inhibitor N-Acetyl-D-lactosamine rescued T cell proliferation. N-Acetyl-D-lactosamine also rescued the proliferation of T cells in prostate-draining lymph nodes of TRAMP mice affected by prostate intraepithelial neoplasia. Additionally, Gal-3 impacted prostate CSC tumorigenic and metastatic potential in vivo, as Gal-3 silencing in prostate CSCs reduced both primary tumor growth and secondary invasion. Gal-3 was also found expressed in more differentiated prostate cancer cells, but with different intracellular distribution as compared to CSCs, which suggests different functions of Gal-3 in the two cell populations. In fact, the prevalent nuclear and cytoplasmic distribution of Gal-3 in prostate CSCs made them less susceptible to apoptosis, when compared to more differentiated prostate cancer cells, in which Gal-3 was predominantly intra-cytoplasmic. Finally, we found Gal-3 expressed in human and mouse prostate intraepithelial neoplasia lesions and in metastatic lymph nodes. All together, these findings identify Gal-3 as a key molecule and a potential therapeutic target already in the early phases of prostate cancer progression and metastasis.
Vibiro vulnificus hemolysin associates with gangliosides.[Pubmed:32228455]
BMC Microbiol. 2020 Mar 30;20(1):69.
BACKGROUND: Vibrio vulnificus hemolysin (VVH) is a pore-forming toxin secreted by Vibrio vulnificus. Cellular cholesterol was believed to be the receptor for VVH, because cholesterol could bind to VVH and preincubation with cholesterol inhibited cytotoxicity. It has been reported that specific glycans such as N-acetyl-D-galactosamine and N-Acetyl-D-lactosamine bind to VVH, however, it has not been known whether these glycans could inhibit the cytotoxicity of VVH without oligomer formation. Thus, to date, binding mechanisms of VVH to cellular membrane, including specific receptors have not been elucidated. RESULTS: We show here that VVH associates with ganglioside GM1a, Fucosyl-GM1, GD1a, GT1c, and GD1b by glycan array. Among them, GM1a could pulldown VVH. Moreover, the GD1a inhibited the cytotoxicity of VVH without the formation of oligomers. CONCLUSION: This is the first report of a molecule able to inhibit the binding of VVH to target cells without oligomerization of VVH.
Novel transglycosylation activity of beta-N-acetylglucosaminidase of Lecanicillium lecanii produced by submerged culture.[Pubmed:31887380]
Int J Biol Macromol. 2020 Feb 15;145:759-767.
N-acetylglucosaminidase produced from Lecanicillium lecanii on submerged culture displayed hydrolytic and transglycosylation activities. The highest specific activity for the enzyme was 1.87 U/mg after 120 h of culture. The chromatographic purification for a single protein fraction showed a molecular weight of 50.4 kDa and hydrolytic N-acetylglucosaminidase activity of 17.59 U/mg at 37 degrees C and pH 6. This enzyme was able to transglycosylate and to synthesize oligosaccharides from 2 to 6 units with a degree of acetylation between 100 and 26% employing glucose, mannose, N-acetyl-D-glucosamine and N-Acetyl-D-lactosamine as donor substrates. Optimal conditions of temperature and pH were determined for both types of enzymatic activities.
Surface display of sialyltransferase on the outer membrane of Escherichia coli and ClearColi.[Pubmed:31186105]
Enzyme Microb Technol. 2019 Sep;128:1-8.
alpha2,3-Sialyltransferase from Pasteurella multocida (PmST1) is an enzyme that transfers a sialyl group of donor substrates to an acceptor substrate called N-Acetyl-D-lactosamine (LacNAc). In this study PmST1 was expressed on the outer membrane of wildtype Escherichia coli (BL21) with lipopolysaccharide (LPS) and ClearColi with no LPS, and then the enzyme activity and expression level of PmST1 were compared. As the first step, the expression levels of PmST1 on the outer membranes of wildtype E. coli (BL21) and ClearColi were compared according to the IPTG induction time, and the absolute amount of surface-displayed PmST1 was calculated using densitometry of SDS-PAGE. As the next step, the influence of LPS on the PmST1 activity was estimated by analyzing Michaelis-Menten plot. The enzyme activity of PmST1 was analyzed by measuring the concentration of CMP, which was a by-product after the transfer of the sialyl group of donor compounds to the acceptor compounds. From a Michaelis-Menten plot, the enzyme activity of the surface-displayed PmST1 and the maximum rate (V(max)) of ClearColi were higher than those of wildtype E. coli (BL21). However, the K(M) value, which represented the concentration of substrate to reach half the maximum rate (V(max)), was similar for both enzymes. These results represented such a difference in enzyme activity was occurred from the interference of LPS on the mass transport of the donor and acceptor to PmST1 for the sialyl group transfer.
Structure of the zebrafish galectin-1-L2 and model of its interaction with the infectious hematopoietic necrosis virus (IHNV) envelope glycoprotein.[Pubmed:30834446]
Glycobiology. 2019 May 1;29(5):419-430.
Galectins, highly conserved beta-galactoside-binding lectins, have diverse regulatory roles in development and immune homeostasis and can mediate protective functions during microbial infection. In recent years, the role of galectins in viral infection has generated considerable interest. Studies on highly pathogenic viruses have provided invaluable insight into the participation of galectins in various stages of viral infection, including attachment and entry. Detailed mechanistic and structural aspects of these processes remain undetermined. To address some of these gaps in knowledge, we used Zebrafish as a model system to examine the role of galectins in infection by infectious hematopoietic necrosis virus (IHNV), a rhabdovirus that is responsible for significant losses in both farmed and wild salmonid fish. Like other rhabdoviruses, IHNV is characterized by an envelope consisting of trimers of a glycoprotein that display multiple N-linked oligosaccharides and play an integral role in viral infection by mediating the virus attachment and fusion. Zebrafish's proto-typical galectin Drgal1-L2 and the chimeric-type galectin Drgal3-L1 interact directly with the glycosylated envelope of IHNV, and significantly reduce viral attachment. In this study, we report the structure of the complex of Drgal1-L2 with N-Acetyl-D-lactosamine at 2.0 A resolution. To gain structural insight into the inhibitory effect of these galectins on IHNV attachment to the zebrafish epithelial cells, we modeled Drgal3-L1 based on human galectin-3, as well as, the ectodomain of the IHNV glycoprotein. These models suggest mechanisms for which the binding of these galectins to the IHNV glycoprotein hinders with different potencies the viral attachment required for infection.
Galectin-3 mediates pulmonary vascular remodeling in hypoxia-induced pulmonary arterial hypertension.[Pubmed:28826890]
J Am Soc Hypertens. 2017 Oct;11(10):673-683.e3.
Pulmonary vascular adventitia serves as a key regulator of pulmonary vascular remodeling in the pathogenesis of pulmonary arterial hypertension (PAH). Excessive proliferation and differentiation of pulmonary adventitial fibroblasts (PAFs) are proven to be crucial in the pathogenesis of PAH. Galectin-3 (Gal-3) is known as a key fibroblasts activating factor which is involved in the fibrogenesis of several diseases, such as pulmonary fibrosis, vascular fibrosis, and heart failure. Therefore, we seek to investigate the potential role of Gal-3 in regulating PAF cells in the pathogenesis of PAH. Gal-3 plasma concentration was significantly higher in PAH patients. Gal-3 was upregulated in pulmonary artery adventitia of hypoxia-induced PAH rats. Inhibition of Gal-3 with N-Acetyl-D-lactosamine (N-Lac) ameliorated PAH and pulmonary vascular remodeling. Gal-3 can stimulate the proliferation, differentiation, and collagen synthesis of PAFs, which was reversed by N-Lac. Transforming growth factor beta1 increased Gal-3 expression in PAFs, whereas N-Lac significantly suppressed transforming growth factor beta1-induced proliferation, differentiation, and collagen synthesis of PAFs. Gal-3 serves as a critical regulator in the pathogenesis of PAH by regulating the proliferation, differentiation, and extracellular matrix deposition synthesis of PAFs. Inhibition of Gal-3 may represent a novel therapeutic target for PAH treatment.
Selection and identification of specific glycoproteins and glycan biomarkers of macrophages involved in Mycobacterium tuberculosis infection.[Pubmed:28454656]
Tuberculosis (Edinb). 2017 May;104:95-106.
Macrophages are the primary host target cells of Mycobacterium tuberculosis (M.tb). However, little is known about the changes of membrane glycopatterns of macrophages in response to M. tb infection. Using lectin microarrays we compared the differential expression of glycopatterns of macrophages upon stimulation with the heat-inactivated virulent M.tb H37Rv or attenuate M.tb H37Ra. We found that widespread alteration of macrophage membrane glycopatterns were induced by the heat-inactivated virulent M. tb H37Rv, as shown by the significantly changed binding abilities of 11 lectins (sugar binding proteins) among 40 lectins tested. The binding ability of the lectin ABA to macrophages showed the greatest increase after virulent M. tb H37Rv treatment, which suggests that the expression of N-Acetyl-D-lactosamine (ABA binding ligand Galbeta1-3GalNAc, O-link glycan) is mainly increased on macrophages during virulent M.tb infection. Addition of ABA blocked the attachment/engulfment of M. tb H37Rv, but not H37Ra, to macrophages. Further, increased glycosylated CD44, one of ABA-binding glycoproteins on macrophages, was identified by pull-down assays with ABA-agarose, followed by mass spectrometry and western blotting. ABA directly binds with Galbeta1-3GalNAc-glycosylated CD44 on macrophage, and inhibits M. tb mannose-capped lipoarabinomannan (ManLAM) binding to glycosylated CD44. Moreover, ABA increases IL-6, but reduces IL-10 production of ManLAM-treated macrophages and inhibits M. tb H37Rv-induced necrosis in macrophages. Our study will help to reveal the mechanism of pathogenicity and virulence of M. tb from a new perspective and provide a potential new diagnostic and therapeutic strategy for tuberculosis based on glycopatterns, ABA and its ligand Galbeta1-3GalNAc-glycosylated CD44 target molecule on macrophage.
Association of Cardiac Galectin-3 Expression, Myocarditis, and Fibrosis in Chronic Chagas Disease Cardiomyopathy.[Pubmed:28322201]
Am J Pathol. 2017 May;187(5):1134-1146.
Chronic Chagas disease cardiomyopathy, caused by Trypanosoma cruzi infection, is a major cause of heart failure in Latin America. Galectin-3 (Gal-3) has been linked to cardiac remodeling and poor prognosis in heart failure of different etiologies. Herein, we investigated the involvement of Gal-3 in the disease pathogenesis and its role as a target for disease intervention. Gal-3 expression in mouse hearts was evaluated during T. cruzi infection by confocal microscopy and flow cytometry analysis, showing a high expression in macrophages, T cells, and fibroblasts. In vitro studies using Gal-3 knockdown in cardiac fibroblasts demonstrated that Gal-3 regulates cell survival, proliferation, and type I collagen synthesis. In vivo blockade of Gal-3 with N-Acetyl-D-lactosamine in T. cruzi-infected mice led to a significant reduction of cardiac fibrosis and inflammation in the heart. Moreover, a modulation in the expression of proinflammatory genes in the heart was observed. Finally, histological analysis in human heart samples obtained from subjects with Chagas disease who underwent heart transplantation showed the expression of Gal-3 in areas of inflammation, similar to the mouse model. Our results indicate that Gal-3 plays a role in the pathogenesis of experimental chronic Chagas disease, favoring inflammation and fibrogenesis. Moreover, by demonstrating Gal-3 expression in human hearts, our finding reinforces that this protein could be a novel target for drug development for Chagas cardiomyopathy.
Two N-terminally truncated variants of human beta-galactoside alpha2,6 sialyltransferase I with distinct properties for in vitro protein glycosylation.[Pubmed:27102286]
Glycobiology. 2016 Oct;26(10):1097-1106.
Sialic acid groups of protein N-glycans are important determinants of biological activity. Exposed at the end of the glycan chain, they are potential targets for glycan remodeling. Sialyltransferases (STs; EC 2.4.99) are the enzymes that catalyze the sialic acid transfer from a CMP-activated donor on to a carbohydrate acceptor in vivo. Recombinant expression of the full-length human beta-galactoside alpha2,6 sialyltransferase I (ST6Gal-I) was hampered and therefore variants with truncated N-termini were investigated. We report on the distinct properties of two N-terminally truncated versions of ST6Gal-I, namely Delta89ST6Gal-I and Delta108ST6Gal-I, which were successfully expressed in human embryonic kidney cells. The different properties of these enzymes result most probably from the loss of interactions from helix alpha1 in the Delta108ST6Gal-I variant, which plays a role in acceptor substrate binding. The K(m) for N-Acetyl-D-lactosamine was 10-fold increased for Delta108ST6Gal-I (84 mM) as compared to Delta89ST6Gal-I (8.3 mM). The two enzyme variants constitute a suitable tool box for the terminal modification of N-glycans. While the enzyme Delta89ST6Gal-I exhibited both ST (di-sialylation) and sialidase activity on a monoclonal antibody, the enzyme Delta108ST6Gal-I showed only ST activity with specificity for mono-sialylation.
Macrophages and galectin 3 play critical roles in CVB3-induced murine acute myocarditis and chronic fibrosis.[Pubmed:26002282]
J Mol Cell Cardiol. 2015 Aug;85:58-70.
Macrophage influx and galectin 3 production have been suggested as major players driving acute inflammation and chronic fibrosis in many diseases. However, their involvement in the pathogenesis of viral myocarditis and subsequent cardiomyopathy are unknown. Our aim was to characterise the role of macrophages and galectin 3 on survival, clinical course, viral burden, acute pathology, and chronic fibrosis in coxsackievirus B3 (CVB3)-induced myocarditis. Our results showed that C3H/HeJ mice infected with CVB3 and depleted of macrophages by liposome-encapsulated clodronate treatment compared with infected untreated mice presented higher viral titres but reduced acute myocarditis and chronic fibrosis, compared with untreated infected mice. Increased galectin 3 transcriptional and translational expression levels correlated with CVB3 infection in macrophages and in non-depleted mice. Disruption of the galectin 3 gene did not affect viral titres but reduced acute myocarditis and chronic fibrosis compared with C57BL/6J wild-type mice. Similar results were observed after pharmacological inhibition of galectin 3 with N-Acetyl-D-lactosamine in C3H/HeJ mice. Our results showed a critical role of macrophages and their galectin 3 in controlling acute viral-induced cardiac injury and the subsequent fibrosis. Moreover, the fact that pharmacological inhibition of galectin 3 induced similar results to macrophage depletion regarding the degree of acute cardiac inflammation and chronic fibrosis opens up the possibility of new pharmacological strategies for viral myocarditis.
Glycan specificity of the Vibrio vulnificus hemolysin lectin outlines evolutionary history of membrane targeting by a toxin family.[Pubmed:24862282]
J Mol Biol. 2014 Jul 29;426(15):2800-12.
Pore-forming toxins (PFTs) are a class of pathogen-secreted molecules that oligomerize to form transmembrane channels in cellular membranes. Determining the mechanism for how PFTs bind membranes is important in understanding their role in disease and for developing possible ways to block their action. Vibrio vulnificus, an aquatic pathogen responsible for severe food poisoning and septicemia in humans, secretes a PFT called V. vulnificus hemolysin (VVH), which contains a single C-terminal targeting domain predicted to resemble a beta-trefoil lectin fold. In order to understand the selectivity of the lectin for glycan motifs, we expressed the isolated VVH beta-trefoil domain and used glycan-chip screening to identify that VVH displays a preference for terminal galactosyl groups including N-acetyl-d-galactosamine and N-Acetyl-D-lactosamine. The X-ray crystal structure of the VVH lectin domain solved to 2.0A resolution reveals a heptameric ring arrangement similar to the oligomeric form of the related, but inactive, lectin from Vibrio cholerae cytolysin. Structures bound to glycerol, N-acetyl-d-galactosamine, and N-Acetyl-D-lactosamine outline a common and versatile mode of recognition allowing VVH to target a wide variety of cell-surface ligands. Sequence analysis in light of our structural and functional data suggests that VVH may represent an earlier step in the evolution of Vibrio PFTs.
Ligand binding and complex formation of galectin-3 is modulated by pH variations.[Pubmed:24147723]
Biochem J. 2014 Jan 1;457(1):107-15.
Galectin-3-dependent clusters or lattices are formed at the surface as well as in distinct organelles of eukaryotic cells. Incorporation into membrane proximal networks can fix glycoproteins within subcellular domains or sort them into distinct transport pathways. In the present paper we analysed the effect of acidification on the sugar binding and self-oligomerization of galectin-3. Using a fluorescence anisotropy assay we measured decreasing galectin-3 affinities to the blood group antigen GalNAcalpha1-3(Fucalpha1-2)Galbeta1-4Glc under low pH conditions. Binding to the strong interaction partner N-Acetyl-D-lactosamine was also lost at pH 5.0, whereas the less efficient ligand lactose was still able to bind. This indicates that variations in the binding specificity to distinct glycans can be observed by altering the pH. The formation of galectin-3-based complexes by interaction with the multivalent glycoproteins asialofetuin or transferrin was also obliterated at acidic pH and the ligand-binding affinity itself was modulated by oligomerization of the lectin. When galectin-3 was added to giant plasma membrane vesicles from the apical surface of epithelial cells, pH modulation could generate or eliminate the formation of membrane domains enriched with p75(NTR) (neurotrophin receptor p75). In conclusion, the results of the present study suggest that the formation and composition of galectin-3 networks can be fine-tuned by changes in the environmental pH.
Purification of Colocasia esculenta lectin and determination of its anti-insect potential towards Bactrocera cucurbitae.[Pubmed:24006804]
J Environ Biol. 2013 Jan;34(1):31-6.
The present study reports the purification of a lectin from Colocasia esculenta (L.) Schott corms and evaluation of its anti-insect potential towards Bactrocera cucurbitae (Coquilett). The lectin was found to be specific towards N-Acetyl-D-lactosamine (LacNac), a disaccharide and asialofetuin, a desialylated serum glycoprotein in hemagglutination inhibition assay. Asialofetuin was used as a ligand to purify Colocasia esculenta agglutinin (CEA) by affinity chromatography. The purity of CEA was ascertained by the presence of a single band in reducing SDS-PAGE at pH 8.3. The affinity purified CEA was employed in artificial diet bioassay of second instar larvae (64-72 hr old) of the B. cucurbitae at concentrations ranging between 10-160 microg ml(-1). The lectin significantly (p < 0.01) decreased the percent pupation and emergence with respect to control. Effect on various enzymes was studied by employing LC50 (51.6 microg ml(-1)) CEA in the artificial diet bioassay of second instar larvae. All the enzymes tested namely esterases, phosphatases (acid and alkaline), superoxide dismutases, catalase and glutathione-S-transferase showed a significant (p < 0.01, p < 0.05) increase in their enzyme and specific activities. These results showed that CEA affected normal growth and development and presented stress to the larvae, activating their detoxification and anti-oxidant systems. Thus, the lectin seems to be a useful candidate for the control measures of B. cucurbitae under the integrated pest management (IPM) system.


