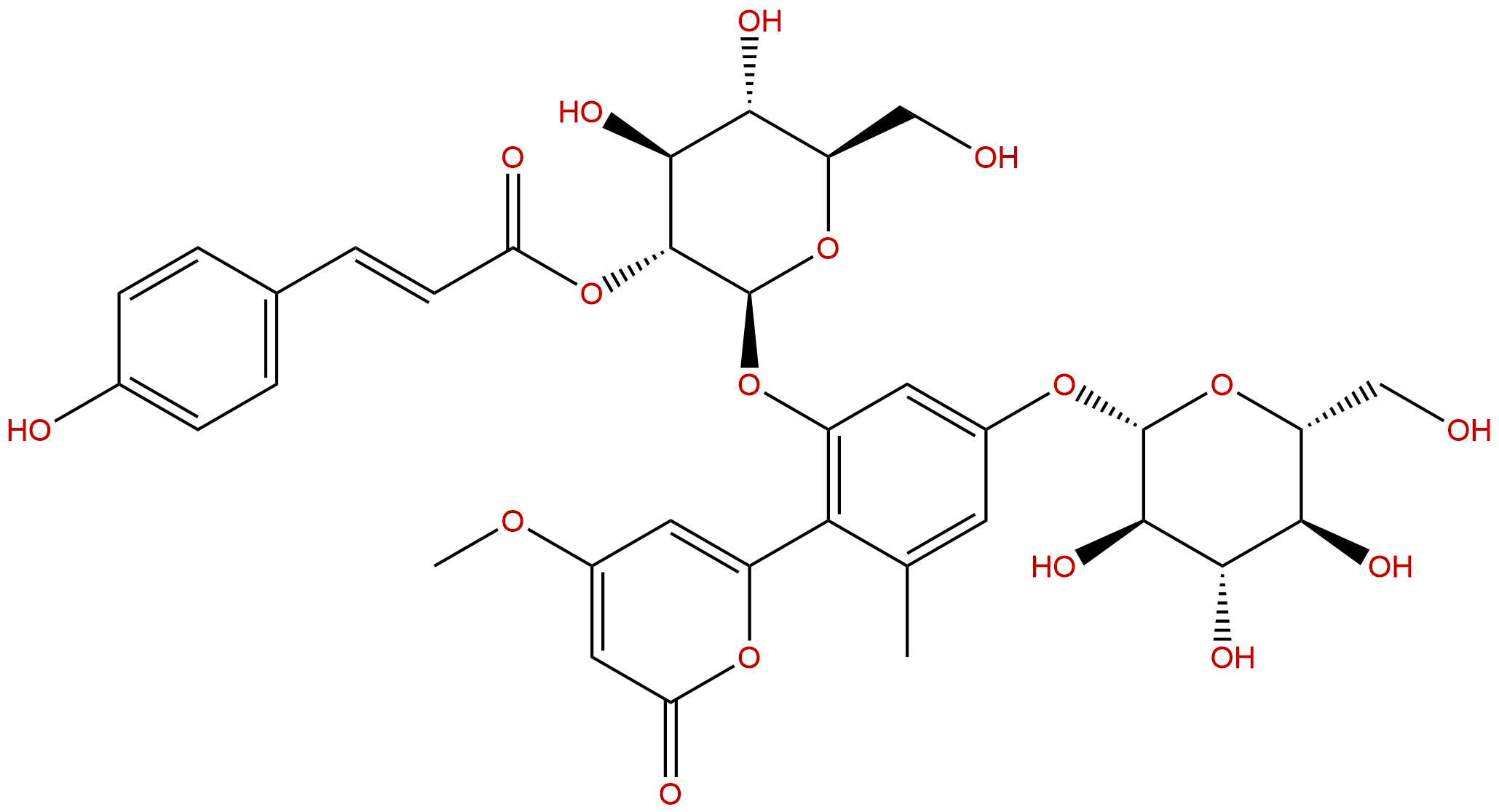
Aloenin BCAS# 106533-41-9 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 106533-41-9 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C34H38O17 | M.Wt | 718.66 |
| Type of Compound | Anthraquinones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Aloenin B Dilution Calculator

Aloenin B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3915 mL | 6.9574 mL | 13.9148 mL | 27.8296 mL | 34.787 mL |
| 5 mM | 0.2783 mL | 1.3915 mL | 2.783 mL | 5.5659 mL | 6.9574 mL |
| 10 mM | 0.1391 mL | 0.6957 mL | 1.3915 mL | 2.783 mL | 3.4787 mL |
| 50 mM | 0.0278 mL | 0.1391 mL | 0.2783 mL | 0.5566 mL | 0.6957 mL |
| 100 mM | 0.0139 mL | 0.0696 mL | 0.1391 mL | 0.2783 mL | 0.3479 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 7-Oxo-ganoderic acid Z2
Catalog No.:BCX1174
CAS No.:1446104-52-4
- 15-Oxospiramilactone
Catalog No.:BCX1173
CAS No.:1053172-87-4
- Tirotundin 3-O-methyl ether
Catalog No.:BCX1172
CAS No.:1454840-36-8
- Cyaonoside B
Catalog No.:BCX1171
CAS No.:51161-58-1
- Ganolactone A
Catalog No.:BCX1170
CAS No.:173268-82-1
- Guattegaumerine
Catalog No.:BCX1169
CAS No.:21446-35-5
- 25(R)-3β,17α-Dihydroxy-5α-spirostan-6-one 3-O-α-D-rhamnopyranosyl-(1→2)-β-D-glucopyranoside
Catalog No.:BCX1168
CAS No.:143051-94-9
- Hispolon
Catalog No.:BCX1167
CAS No.:173933-40-9
- 6-methoxy-bispyranoxanthone
Catalog No.:BCX1166
CAS No.:115713-10-5
- Aucubigenin
Catalog No.:BCX1165
CAS No.:64274-28-8
- Coronafacic acid
Catalog No.:BCX1164
CAS No.:62251-98-3
- Coronatine
Catalog No.:BCX1163
CAS No.:62251-96-1
- Glucovanillin
Catalog No.:BCX1176
CAS No.:494-08-6
- Halofuginone hydrobromide
Catalog No.:BCX1177
CAS No.:64924-67-0
- 2-Hydroxypinocembrin
Catalog No.:BCX1178
CAS No.:40489-17-6
- 11-Oxo-ganoderic acid DM
Catalog No.:BCX1179
CAS No.:1408244-15-4
- GDP-L-Fuc.2Na
Catalog No.:BCX1180
CAS No.:15839-70-0
- N-Acetyl-D-lactosamine
Catalog No.:BCX1181
CAS No.:32181-59-2
- Acetylarenobufagin
Catalog No.:BCX1182
CAS No.:184673-79-8
- 5-Methoxyafrormosin 7-O-glucoside
Catalog No.:BCX1183
CAS No.:64656-92-4
- Heterophyllin J
Catalog No.:BCX1184
CAS No.:660397-15-9
- 6-Acetyl-7-hydroxy-2,3-dimethylchromone
Catalog No.:BCX1185
CAS No.:225518-70-7
- Neoisoliquiritigenin
Catalog No.:BCX1186
CAS No.:7014-39-3
- Rubropunctamine
Catalog No.:BCX1187
CAS No.:514-66-9
[A novel naphthalene derivative from Aloe barbadensis].[Pubmed:23888696]
Yao Xue Xue Bao. 2013 May;48(5):723-7.
To investigate the chemical constituents of A. barbadensis, aqueous extract of the plant was subjected to preparative medium pressure liquid chromatography (MPLC). The chemical structures were mainly determined by spectroscopic evidences (UV, IR, HR-MS, 1H NMR, 13C NMR, HSQC, 1H-1H COSY and HMBC) and chemical methods. A new O, O, O-triglucosylated naphthalene derivative, together with two known 6-phenyl-2-pyrone derivatives and four 5-methylchromones, were isolated and identified as 1-((3-((4- O-beta-D-glucopyranosyl)-beta-D-xylopyranosyloxymethyl)-1-hydroxy-8-alpha-L-rhamnopyranosyloxy)naphthalene-2-y])-ethanone (1), 10-O-beta-D-glucopyranosyl aloenin (2), Aloenin B (3), aloesin (4), 8-C-glucosyl-(R)-aloesol (5), 8-C-glucosyl-7-O-methyl-(S)-aloesol (6), and isoaloeresin D (7). Compound 1 is a novel naphthalene derivative and named as aloveroside B, compounds 2-3 are isolated from this Aloe species for the first time.
New bioactive compounds from Aloe hijazensis.[Pubmed:19521919]
Nat Prod Res. 2009;23(11):1035-49.
The chemical constituents and biological activities of leaves and roots of Aloe hijazensis, collected in Saudi Arabia, are reported here for the first time. Twenty-two compounds were obtained, among them eight hydroxyquinones: aloe-emodin (1), emodin (2), chrysophanol (3), aloesaponarin II 3-methyl ether (4), ziganein (5), ziganein-5-methyl ether (6a), aloesaponarin I (7) and chrysophanein (8), the dihydro-isocoumarin feralolide (9), 4,7-dichloro-quinoline (10), the triterpene lupeol (11), the anthrone aloin (12), three aloenin derivatives, aloenin (13) ethylidene-aloenin (14), and Aloenin B (15), four flavonoids, quercetin (16), kaempferol (17) cosmosiin (18) and isovitexin (19), and cinnamic acid (20) and two further analogues, caffeic acid (21) and ferulic acid (22). While 15 of the isolated compounds were found in the leaves, 12 were isolated from roots of the plant. Compounds 6a and 10 are reported as new natural constituents, while the compounds 4, 5, 8, and 18 are reported here for the first time from Aloe spp. The structures of the compounds were deduced by intensive studies of their UV, NMR, MS data and by comparison with related structures. The biological activity of plant extracts was studied against various microbial strains, and potent anti-bacterial and anti-fungal activities were found. [image omitted] [image omitted].
The phytochemical profile and identification of main phenolic compounds from the leaf exudate of Aloe secundiflora by high-performance liquid chromatography-mass spectroscopy.[Pubmed:12693631]
Phytochem Anal. 2003 Mar-Apr;14(2):83-6.
The phytochemical profile of Aloe secundiflora (Aloeaceae) and the identity of eight major compounds, including the two main constituents, have been determined from the leaf exudate of this ethnoveterinary used species from Kenya and Tanzania. Analytical HPLC-MS studies of the exudate have revealed that it comprises a mixture of phenolic compounds, mainly anthrones (aloenin, Aloenin B, isobarbaloin, barbaloin and other aloin derivatives), chromones and phenylpyrones with a low content of polysaccharides and aliphatic compounds. The high percentage of anthrones in the exudate could provide a first line of evidence for the use of the plant in ethnoveterinary practices.


