GlucovanillinCAS# 494-08-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

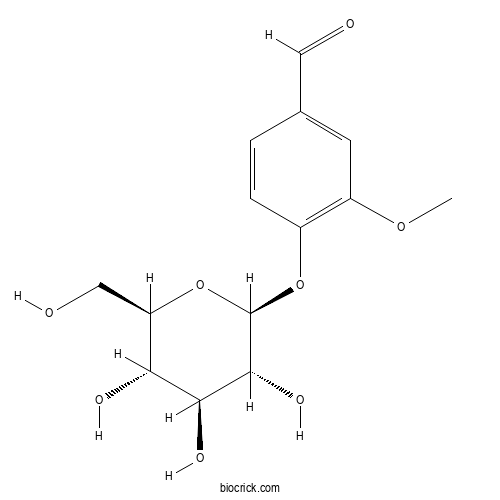
| Cas No. | 494-08-6 | SDF | Download SDF |
| PubChem ID | 6452133.0 | Appearance | Powder |
| Formula | C14H18O8 | M.Wt | 314.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-methoxy-4-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxybenzaldehyde | ||
| SMILES | COC1=C(C=CC(=C1)C=O)OC2C(C(C(C(O2)CO)O)O)O | ||
| Standard InChIKey | LPRNQMUKVDHCFX-RKQHYHRCSA-N | ||
| Standard InChI | InChI=1S/C14H18O8/c1-20-9-4-7(5-15)2-3-8(9)21-14-13(19)12(18)11(17)10(6-16)22-14/h2-5,10-14,16-19H,6H2,1H3/t10-,11-,12+,13-,14-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Glucovanillin Dilution Calculator

Glucovanillin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1818 mL | 15.9089 mL | 31.8177 mL | 63.6355 mL | 79.5444 mL |
| 5 mM | 0.6364 mL | 3.1818 mL | 6.3635 mL | 12.7271 mL | 15.9089 mL |
| 10 mM | 0.3182 mL | 1.5909 mL | 3.1818 mL | 6.3635 mL | 7.9544 mL |
| 50 mM | 0.0636 mL | 0.3182 mL | 0.6364 mL | 1.2727 mL | 1.5909 mL |
| 100 mM | 0.0318 mL | 0.1591 mL | 0.3182 mL | 0.6364 mL | 0.7954 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Aloenin B
Catalog No.:BCX1175
CAS No.:106533-41-9
- 7-Oxo-ganoderic acid Z2
Catalog No.:BCX1174
CAS No.:1446104-52-4
- 15-Oxospiramilactone
Catalog No.:BCX1173
CAS No.:1053172-87-4
- Tirotundin 3-O-methyl ether
Catalog No.:BCX1172
CAS No.:1454840-36-8
- Cyaonoside B
Catalog No.:BCX1171
CAS No.:51161-58-1
- Ganolactone A
Catalog No.:BCX1170
CAS No.:173268-82-1
- Guattegaumerine
Catalog No.:BCX1169
CAS No.:21446-35-5
- 25(R)-3β,17α-Dihydroxy-5α-spirostan-6-one 3-O-α-D-rhamnopyranosyl-(1→2)-β-D-glucopyranoside
Catalog No.:BCX1168
CAS No.:143051-94-9
- Hispolon
Catalog No.:BCX1167
CAS No.:173933-40-9
- 6-methoxy-bispyranoxanthone
Catalog No.:BCX1166
CAS No.:115713-10-5
- Aucubigenin
Catalog No.:BCX1165
CAS No.:64274-28-8
- Coronafacic acid
Catalog No.:BCX1164
CAS No.:62251-98-3
- Halofuginone hydrobromide
Catalog No.:BCX1177
CAS No.:64924-67-0
- 2-Hydroxypinocembrin
Catalog No.:BCX1178
CAS No.:40489-17-6
- 11-Oxo-ganoderic acid DM
Catalog No.:BCX1179
CAS No.:1408244-15-4
- GDP-L-Fuc.2Na
Catalog No.:BCX1180
CAS No.:15839-70-0
- N-Acetyl-D-lactosamine
Catalog No.:BCX1181
CAS No.:32181-59-2
- Acetylarenobufagin
Catalog No.:BCX1182
CAS No.:184673-79-8
- 5-Methoxyafrormosin 7-O-glucoside
Catalog No.:BCX1183
CAS No.:64656-92-4
- Heterophyllin J
Catalog No.:BCX1184
CAS No.:660397-15-9
- 6-Acetyl-7-hydroxy-2,3-dimethylchromone
Catalog No.:BCX1185
CAS No.:225518-70-7
- Neoisoliquiritigenin
Catalog No.:BCX1186
CAS No.:7014-39-3
- Rubropunctamine
Catalog No.:BCX1187
CAS No.:514-66-9
- Glycyrrhizic acid methyl ester
Catalog No.:BCX1188
CAS No.:104191-95-9
A comprehensive review of eclectic approaches to the biological synthesis of vanillin and their application towards the food sector.[Pubmed:38440686]
Food Sci Biotechnol. 2024 Jan 3;33(5):1019-1036.
Vanillin, a highly regarded flavor compound, has earned widespread recognition for its natural and aromatic qualities, piquing substantial interest in the scientific community. This comprehensive review delves deeply into the intricate world of vanillin synthesis, encompassing a wide spectrum of methodologies, including enzymatic, microbial, and immobilized systems. This investigation provides a thorough analysis of the precursors of vanillin and also offers a comprehensive overview of its transformation through these diverse processes, making it an invaluable resource for researchers and enthusiasts alike. The elucidation of different substrates such as ferulic acid, eugenol, veratraldehyde, vanillic acid, Glucovanillin, and C(6)-C(3) phenylpropanoids adds a layer of depth and insight to the understanding of vanillin synthesis. Moreover, this comprehensive review explores the multifaceted applications of vanillin within the food industry. While commonly known as a flavoring agent, vanillin transcends this role by finding extensive use in food preservation and food packaging. The review meticulously examines the remarkable preservative properties of vanillin, providing a profound understanding of its crucial role in the culinary and food science sectors, thus making it an indispensable reference for professionals and researchers in these domains.
Nature-inspired synthesis of antibacterial glucovanillin derivatives.[Pubmed:36940919]
Fitoterapia. 2023 Jun;167:105475.
The ongoing threat of Antimicrobial Resistance (AMR) complicated by the rise of Multidrug-Resistant (MDR) pathogens calls for increased efforts in the search for novel treatment options. While deriving inspiration from antibacterial natural compounds, this study aimed at using synthetic approaches to generate a series of Glucovanillin derivatives and explore their antibacterial potentials. Among the synthesized derivatives, optimum antibacterial activities were exhibited by those containing 2,4- and 3,5-dichlorophenylamino group coupled to a Glucovanillin moiety (compounds 6h and 8d respectively). In those compounds, the Minimum Inhibitory Concentrations (MIC) of 128-256 mug/mL were observed against reference and MDR strains of Klebsiella pneumoniae, Methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VRE). Moreover, these findings emphasize the claims from previous reports on the essence of smaller molecular size, the presence of protonatable amino groups and halogens in potential antibacterial agents. The observed moderate and broad-spectrum activities of the stated derivatives point to their suitability as potential leads towards further efforts to improve their antibacterial activities.
Metabolite transformation and beta-d-glucosidase activity during the high hydrostatic pressure assisted curing of vanilla beans (Vanilla planifolia) to improve phenolic compounds formation.[Pubmed:35219994]
Food Chem. 2022 Aug 1;384:132497.
Current methods for vanilla bean curing are long and reduce the enzymatic activity necessary for flavor development. High hydrostatic pressure (HHP) at 50-600 MPa was used to improve phenolic compounds formation and beta-d-glucosidase activity in vanilla beans compared with scalded beans. Phenolics were analyzed by HPLC and beta-d-glucosidase activity by spectrophotometry. Vanillin was the main phenolic and it was formed by beta-d-Glucovanillin hydrolysis and vanillyl alcohol oxidation. HHP improved vanillin content and influenced beta-d-glucosidase activity. At the beginning of the curing the highest increments of vanillin were produced at 400 MPa (up to 15%), while at the end, this was observed at 50 (138%) and 600 MPa (74%). Maximum increment of up to 400% in beta-d-glucosidase activity was observed from 100 to 300 MPa, which was attributed to tissue decompartmentalization, and conformational changes induced by pressure. HHP could be used during vanilla curing to improve vanillin content and beta-d-glucosidase activity.
Producing natural vanilla extract from green vanilla beans using a beta-glucosidase from Alicyclobacillus acidiphilus.[Pubmed:33508335]
J Biotechnol. 2021 Mar 10;329:21-28.
Current methods for the production of natural vanilla extract are long and tedious, and the efficiency of the vanillin extraction is usually conditioned by different factors during the traditional curing process (temperatures and weather conditions). As an important fraction of vanillin is present in the form of Glucovanillin in green beans, endogenous beta-glucosidases contribute to its hydrolysis; however, these enzymes lose efficiency during the curing process. The use of extremophilic organisms as a source of an appropriate exogenous enzyme can offer a valid alternative when producing natural vanillin. Here, a beta-glucosidase from the thermo-acidophilic organism Alicyclobacillus acidiphilus (AacGH1) was cloned, expressed in E. coli BL21, and fully characterized in respect to both function and crystal structure. Notably, AacGH1 was stable at a temperature up to 50 degrees C and exhibited good tolerance to glucose, fructose and organic solvents, in particular it maintained full activity in the presence of up to 20 % (v/v) ethanol. The enzyme was then successfully applied to an ethanol-water (20 % (v/v)) extract of green vanilla beans and the complete hydrolysis of Glucovanillin (1.7 mM) to vanillin, and other flavour compounds commonly found in vanilla, was achieved using 0.5 mg/mL of enzyme in just 15 min at 30 degrees C.
Metabolite Transformation and Enzyme Activities of Hainan Vanilla Beans During Curing to Improve Flavor Formation.[Pubmed:31370187]
Molecules. 2019 Jul 31;24(15):2781.
This paper compares the differences in metabolites of vanilla beans at five different curing stages. Key vanilla flavors, vanillin precursors and main enzymes during the curing process of Hainan vanilla beans were also analyzed. Hundreds of metabolites were detected based on metabolic analyses of a widely targeted metabolome technique, compared with blanched vanilla beans (BVB), sweating vanilla beans (SVB) and drying vanilla beans (DVB), the total peak intensity of cured vanilla beans (CVB) is on the rise. The score plots of principal component analysis indicated that the metabolites were generally similar at the same curing stages, but for the different curing stages, they varied substantially. During processing, vanillin content increased while Glucovanillin content decreased, and vanillic acid was present in sweating beans, but its content was reduced in drying beans. Both p-hydroxybenzaldehyde and p-hydroxybenzoic acid showed the maximum contents in cured beans. Ferulic acid was mainly produced in drying beans and reduced in cured beans. p-coumaric acid increased during the curing process. Vanillyl alcohol in drying beans (0.22%) may be formed by the hydrolysis of glucoside, whose conversion into vanillin may explain its decrease during the curing stage. beta-Glucosidase enzymatic activity was not detected in blanched and sweating beans, but was observed after drying. Peroxidase activity decreased during curing by 94% in cured beans. Polyphenol oxidase activity was low in earlier stages, whereas cellulase activity in processed beans was higher than in green beans, except for cured beans. This study contributes to revealing the formation of flavor components and the biosynthesis pathway of vanillin.
Iron-catalyzed oxidative C-C(vinyl) sigma-bond cleavage of allylarenes to aryl aldehydes at room temperature with ambient air.[Pubmed:30946407]
Chem Commun (Camb). 2019 Apr 18;55(33):4817-4820.
A general and selective iron-catalyzed allylic C-C(vinyl) sigma-bond cleavage of allylarenes without the assistance of heteroatoms to give aryl aldehydes is reported. The unstrained carbon-carbon single bond cleavage reaction uses ambient air as the sole oxidant, proceeds efficiently at room temperature, and allows for exceptional functional-group tolerance, which addresses the long-standing challenges of current C-C bond cleavage/functionalization. Notably, the method enables rapid late-stage oxidation of complex bioactive molecules and can be used to expedite syntheses of natural products (vanillin and Glucovanillin) from readily available chemical feedstocks.
Effect of endogenous and exogenous enzymatic treatment of green vanilla beans on extraction of vanillin and main aromatic compounds.[Pubmed:29892106]
J Food Sci Technol. 2018 Jun;55(6):2059-2067.
Endogenous and exogenous enzymatic hydrolysis carried out to obtain vanilla extracts with higher concentrations of vanillin using green vanilla beans. Sequences initiated with freezing of green vanilla beans at - 1 degrees C for 24 h, followed by endogenous hydrolysis under optimal beta-glucosidase activity at 4.2 and 35 degrees C for 96 h, exogenous hydrolysis with Crystalzyme PML-MX at pH 5.0 and 40 degrees C for 72 h, and ethanol extraction at 40% (v v(-1)) for 30 days. In the proposed method, 200 g of fresh green vanilla beans with 84% moisture (32 g dry base) were used to obtain a liter of single fold vanilla extract. This method allowed the release of 82.57% of the theoretically available vanillin from its precursor Glucovanillin with 5.78 g 100 g(-1) green vanilla beans (dry base). Vanillic acid, p-hydroxybenzaldehyde and vanillyl alcohol were also released and found in commercial and enzymatic extracts. Glucovanillin was detected in commercial and traditional extracts but was absent in enzymatic extracts, indicating incomplete hydrolysis during the curing process. An in vitro assay was conducted to determine if the presence of peroxidase during hydrolysis might affect overall vanillin concentration. Results showed that POD can use vanillin as a substrate under conditions similar to those in which hydrolysis was conducted (pH 5.0 and 50 degrees C), possibly explaining why vanillin concentration was not complete at the end of the process.
Comparative metabolomics in vanilla pod and vanilla bean revealing the biosynthesis of vanillin during the curing process of vanilla.[Pubmed:28587440]
AMB Express. 2017 Dec;7(1):116.
High-performance liquid chromatography-mass spectrometry (LC-MS) was used for comprehensive metabolomic fingerprinting of vanilla fruits prepared from the curing process. In this study, the metabolic changes of vanilla pods and vanilla beans were characterized using MS-based metabolomics to elucidate the biosynthesis of vanillin. The vanilla pods were significantly different from vanilla beans. Seven pathways of vanillin biosynthesis were constructed, namely, Glucovanillin, glucose, cresol, capsaicin, vanillyl alcohol, tyrosine, and phenylalanine pathways. Investigations demonstrated that glucose, cresol, capsaicin, and vanillyl alcohol pathway were detected in a wide range of distribution in microbial metabolism. Thus, microorganisms might have participated in vanillin biosynthesis during vanilla curing. Furthermore, the ion strength of Glucovanillin was stable, which indicated that Glucovanillin only participated in the vanillin biosynthesis during the curing of vanilla.
A re-evaluation of the final step of vanillin biosynthesis in the orchid Vanilla planifolia.[Pubmed:28411481]
Phytochemistry. 2017 Jul;139:33-46.
A recent publication describes an enzyme from the vanilla orchid Vanilla planifolia with the ability to convert ferulic acid directly to vanillin. The authors propose that this represents the final step in the biosynthesis of vanillin, which is then converted to its storage form, Glucovanillin, by glycosylation. The existence of such a "vanillin synthase" could enable biotechnological production of vanillin from ferulic acid using a "natural" vanilla enzyme. The proposed vanillin synthase exhibits high identity to cysteine proteases, and is identical at the protein sequence level to a protein identified in 2003 as being associated with the conversion of 4-coumaric acid to 4-hydroxybenzaldehyde. We here demonstrate that the recombinant cysteine protease-like protein, whether expressed in an in vitro transcription-translation system, E. coli, yeast, or plants, is unable to convert ferulic acid to vanillin. Rather, the protein is a component of an enzyme complex that preferentially converts 4-coumaric acid to 4-hydroxybenzaldehyde, as demonstrated by the purification of this complex and peptide sequencing. Furthermore, RNA sequencing provides evidence that this protein is expressed in many tissues of V. planifolia irrespective of whether or not they produce vanillin. On the basis of our results, V. planifolia does not appear to contain a cysteine protease-like "vanillin synthase" that can, by itself, directly convert ferulic acid to vanillin. The pathway to vanillin in V. planifolia is yet to be conclusively determined.
Contribution of Bacillus Isolates to the Flavor Profiles of Vanilla Beans Assessed through Aroma Analysis and Chemometrics.[Pubmed:26473810]
Molecules. 2015 Oct 9;20(10):18422-36.
Colonizing Bacillus in vanilla (Vanilla planifolia Andrews) beans is involved in Glucovanillin hydrolysis and vanillin formation during conventional curing. The flavor profiles of vanilla beans under Bacillus-assisted curing were analyzed through gas chromatography-mass spectrometry, electronic nose, and quantitative sensory analysis. The flavor profiles were analytically compared among the vanilla beans under Bacillus-assisted curing, conventional curing, and non-microorganism-assisted curing. Vanilla beans added with Bacillus vanillea XY18 and Bacillus subtilis XY20 contained higher vanillin (3.58%+/-0.05% and 3.48%+/-0.10%, respectively) than vanilla beans that underwent non-microorganism-assisted curing and conventional curing (3.09%+/-0.14% and 3.21%+/-0.15%, respectively). Forty-two volatiles were identified from endogenous vanilla metabolism. Five other compounds were identified from exogenous Bacillus metabolism. Electronic nose data confirmed that vanilla flavors produced through the different curing processes were easily distinguished. Quantitative sensory analysis confirmed that Bacillus-assisted curing increased vanillin production without generating any unpleasant sensory attribute. Partial least squares regression further provided a correlation model of different measurements. Overall, we comparatively analyzed the flavor profiles of vanilla beans under Bacillus-assisted curing, indirectly demonstrated the mechanism of vanilla flavor formation by microbes.
Involvement of Colonizing Bacillus Isolates in Glucovanillin Hydrolysis during the Curing of Vanilla planifolia Andrews.[Pubmed:25979899]
Appl Environ Microbiol. 2015 Aug;81(15):4947-54.
Vanilla beans were analyzed using biochemical methods, which revealed that Glucovanillin disperses from the inner part to the outer part of the vanilla bean during the curing process and is simultaneously hydrolyzed by beta-d-glucosidase. Enzymatic hydrolysis was found to occur on the surface of the vanilla beans. Transcripts of the beta-d-glucosidase gene (bgl) of colonizing microorganisms were detected. The results directly indicate that colonizing microorganisms are involved in Glucovanillin hydrolysis. Phylogenetic analysis based on 16S rRNA gene sequences showed that the colonizing microorganisms mainly belonged to the Bacillus genus. bgl was detected in all the isolates and presented clustering similar to that of the isolate taxonomy. Furthermore, inoculation of green fluorescent protein-tagged isolates showed that the Bacillus isolates can colonize vanilla beans. Glucovanillin was metabolized as the sole source of carbon in a culture of the isolates within 24 h. These isolates presented unique Glucovanillin degradation capabilities. Vanillin was the major volatile compound in the culture. Other compounds, such as alpha-cubebene, beta-pinene, and guaiacol, were detected in some isolate cultures. Colonizing Bacillus isolates were found to hydrolyze Glucovanillin in culture, indirectly demonstrating the involvement of colonizing Bacillus isolates in Glucovanillin hydrolysis during the vanilla curing process. Based on these results, we conclude that colonizing Bacillus isolates produce beta-d-glucosidase, which mediates Glucovanillin hydrolysis and influences flavor formation.
Two new compounds from the dry leaves of Pleioblastus amarus (Keng) keng f.[Pubmed:25253092]
J Asian Nat Prod Res. 2014;16(9):930-5.
Two new compounds, xylitol 1-O-(6'-O-p-hydroxylbenzoyl)-glucopyranoside (1) and bambulignan B (2), together with three known ones gastrodin (3), Glucovanillin (4), and rel-(7S,7'R,8R,8'S)-4,4'-dihydroxy-3,3',5,5'-tetramethoxy-7,7'-epoxyligna-9,9'-diol-9(or)9'-O-beta-glucopyranoside (5), were isolated from the 95% EtOH extract of the dry leaves of Pleioblastus amarus (Keng) keng f. Their structures were determined by UV, IR, HR-ESI-MS, CD, and 1D and 2D NMR data analyses as well as GC experiments.
Optimized production of vanillin from green vanilla pods by enzyme-assisted extraction combined with pre-freezing and thawing.[Pubmed:24556615]
Molecules. 2014 Feb 19;19(2):2181-98.
Production of vanillin from natural green vanilla pods was carried out by enzyme-assisted extraction combined with pre-freezing and thawing. In the first step the green vanilla pods were pre-frozen and then thawed to destroy cellular compartmentation. In the second step pectinase from Aspergillus niger was used to hydrolyze the pectin between the Glucovanillin substrate and beta-glucosidase. Four main variables, including enzyme amount, reaction temperature, time and pH, which were of significance for the vanillin content were studied and a central composite design (CCD) based on the results of a single-factor tests was used. Response surface methodology based on CCD was employed to optimize the combination of enzyme amount, reaction temperature, time, and pH for maximum vanillin production. This resulted in the optimal condition in regards of the enzyme amount, reaction temperature, time, and pH at 84.2 mg, 49.5 degrees C, 7.1 h, and 4.2, respectively. Under the optimal condition, the experimental yield of vanillin was 4.63% +/- 0.11% (dwb), which was in good agreement with the value predicted by the model. Compared to the traditional curing process (1.98%) and viscozyme extract (2.36%), the optimized method for the vanillin production significantly increased the yield by 133.85% and 96%, respectively.
Exploration of Vanilla pompona from the Peruvian Amazon as a potential source of vanilla essence: quantification of phenolics by HPLC-DAD.[Pubmed:23265471]
Food Chem. 2013 May 1;138(1):161-7.
This study provides the first chemical investigation of wild-harvested fruits of Vanilla pompona ssp. grandiflora (Lindl.) Soto-Arenas developed in their natural habitat in the Peruvian Amazon. Flowers were hand-pollinated and the resulting fruits were analysed at different developmental stages using an HPLC-DAD method validated for the quantification of Glucovanillin and seven other compounds. The method showed satisfactory linearity (r(2)>0.9969), precision (coefficient of variation <2%), recoveries (70-100%), limit of detection (0.008-0.212 mug/ml), and limit of quantification (0.027-0.707 mug/ml). The evaluation of crude and enzyme-hydrolyzed Soxhlet-extracted samples confirmed the leading role of glucosides in fruit development. LC-ESI-MS studies corroborated the identities of four glucosides and seven aglycones, among them vanillin (5.7/100 g), 4-hydroxybenzyl alcohol (3.6/100 g), and anisyl alcohol (7.1/100 g) were found in high concentrations. The attractive flavor/aroma profile exhibited by wild V. pompona fruits supports studies focused on the development of this species as a specialty crop.
Characterization of a novel beta-thioglucosidase CpTGG1 in Carica papaya and its substrate-dependent and ascorbic acid-independent O-beta-glucosidase activity.[Pubmed:20883440]
J Integr Plant Biol. 2010 Oct;52(10):879-90.
Plant thioglucosidases are the only known S-glycosidases in the large superfamily of glycosidases. These enzymes evolved more recently and are distributed mainly in Brassicales. Thioglucosidase research has focused mainly on the cruciferous crops due to their economic importance and cancer preventive benefits. In this study, we cloned a novel myrosinase gene, CpTGG1, from Carica papaya Linnaeus. and showed that it was expressed in the aboveground tissues in planta. The recombinant CpTGG1 expressed in Pichia pastoris catalyzed the hydrolysis of both sinigrin and glucotropaeolin (the only thioglucoside present in papaya), showing that CpTGG1 was indeed a functional myrosinase gene. Sequence alignment analysis indicated that CpTGG1 contained all the motifs conserved in functional myrosinases from crucifers, except for two aglycon-binding motifs, suggesting substrate priority variation of the non-cruciferous myrosinases. Using sinigrin as substrate, the apparent K(m) and V(max) values of recombinant CpTGG1 were 2.82 mM and 59.9 mumol min(-)(1) mg protein(-)(1) , respectively. The K(cat) /K(m) value was 23 s(-)(1) mM(-)(1) . O-beta-glucosidase activity towards a variety of substrates were tested, CpTGG1 displayed substrate-dependent and ascorbic acid-independent O-beta-glucosidase activity towards 2-nitrophenyl-beta-D-glucopyranoside and 4-nitrophenyl-beta-D-glucopyranoside, but was inactive towards Glucovanillin and n-octyl-beta-D-glucopyranoside. Phylogenetic analysis indicated CpTGG1 belongs to the MYR II subfamily of myrosinases.


