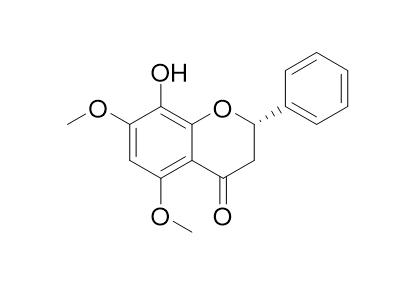
8-Hydroxy-5,7-dimethoxyflavanoneCAS# 201230-40-2 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 201230-40-2 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C17H16O5 | M.Wt | 300.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

8-Hydroxy-5,7-dimethoxyflavanone Dilution Calculator

8-Hydroxy-5,7-dimethoxyflavanone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.33 mL | 16.65 mL | 33.3 mL | 66.6001 mL | 83.2501 mL |
| 5 mM | 0.666 mL | 3.33 mL | 6.66 mL | 13.32 mL | 16.65 mL |
| 10 mM | 0.333 mL | 1.665 mL | 3.33 mL | 6.66 mL | 8.325 mL |
| 50 mM | 0.0666 mL | 0.333 mL | 0.666 mL | 1.332 mL | 1.665 mL |
| 100 mM | 0.0333 mL | 0.1665 mL | 0.333 mL | 0.666 mL | 0.8325 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ganosinensic acid C
Catalog No.:BCX0061
CAS No.:2231756-23-1
- Oxyphyllol B
Catalog No.:BCX0060
CAS No.:226546-99-2
- Nomilinic acid
Catalog No.:BCX0059
CAS No.:35930-20-2
- Ephedrannin A
Catalog No.:BCX0058
CAS No.:82001-39-6
- Ganoderic acid GS-1
Catalog No.:BCX0057
CAS No.:1206781-64-7
- Yakuchinone A
Catalog No.:BCX0056
CAS No.:78954-23-1
- Tectorigenin 7-O-gentiobioside
Catalog No.:BCX0055
CAS No.:67604-94-8
- Peltatoside 7-O-beta-glucopyranoside
Catalog No.:BCX0054
CAS No.:813466-12-5
- Protocatechuic acid 4-O-beta-glucoside
Catalog No.:BCX0053
CAS No.:7361-59-3
- Methyl nomilinate
Catalog No.:BCX0052
CAS No.:77887-51-5
- 1-Phenylethyl beta-D-glucoside
Catalog No.:BCX0051
CAS No.:93199-03-2
- Ganoderiol D
Catalog No.:BCX0050
CAS No.:114567-45-2
- Methyl ganoderate E
Catalog No.:BCX0063
CAS No.:98718-43-5
- Methyl ganoderate F
Catalog No.:BCX0064
CAS No.:98665-08-8
- Schisphenlignan I
Catalog No.:BCX0065
CAS No.:1542234-14-9
- Methyl ganoderate D
Catalog No.:BCX0066
CAS No.:97210-12-3
- Mahuannin E
Catalog No.:BCX0067
CAS No.:1173887-70-1
- Resinacein D
Catalog No.:BCX0068
CAS No.:2231061-47-3
- 1-O-Feruloylglucose
Catalog No.:BCX0069
CAS No.:64625-37-2
- 11alpha-hydroxy-3,7-dioxo-5alpha-lanosta-8,24(E)-dien-26-oic acid
Catalog No.:BCX0070
CAS No.:1245703-70-1
- Clinopodic acid E
Catalog No.:BCX0071
CAS No.:159736-38-6
- N-Methyltetrahydrocolumbamine
Catalog No.:BCX0072
CAS No.:92758-34-4
- Iristectorin A-6''-O-glucoside
Catalog No.:BCX0073
CAS No.:86849-71-0
- Abiesinol B
Catalog No.:BCX0074
CAS No.:1190070-89-3
A methoxyflavanone derivative from the Asian medicinal herb (Perilla frutescens) induces p53-mediated G(2)/M cell cycle arrest and apoptosis in A549 human lung adenocarcinoma.[Pubmed:28710570]
Cytotechnology. 2018 Jun;70(3):899-912.
Perilla frutescens is an Asian dietary herb consumed as an essential seasoning in Japanese cuisine as well as used for a Chinese medicine. Here, we report that a newly found methoxyflavanone derivative from P. frutescens (Perilla-derived methoxyflavanone, PDMF; 8-Hydroxy-5,7-dimethoxyflavanone) shows carcinostatic activity on human lung adenocarcinoma, A549. We found that treatment with PDMF significantly inhibited cell proliferation and decreased viability through induction of G(2)/M cell cycle arrest and apoptosis. The PDMF stimulation induces phosphorylation of tumor suppressor p53 on Ser15, and increases its protein amount in conjunction with up-regulation of downstream cyclin-dependent kinase inhibitor p21(Cip1/Waf1) and proapoptotic caspases, caspase-9 and caspase-3. We also found that small interfering RNA knockdown of p53 completely abolished the PDMF-induced G(2)/M cell cycle arrest, and substantially abrogated its proapoptotic potency. These results suggest that PDMF represents a useful tumor-preventive phytochemical that triggers p53-driven G(2)/M cell cycle arrest and apoptosis.
A flavanone derivative from the Asian medicinal herb (Perilla frutescens) potently suppresses IgE-mediated immediate hypersensitivity reactions.[Pubmed:27986566]
Biochem Biophys Res Commun. 2017 Jan 29;483(1):674-679.
Perilla frutescens is a dietary leafy herb consumed as a traditional Japanese condiment as well as used for Chinese medicine with anti-inflammatory activity. Here we report a hitherto-unrecognized P. frutescens phytochemical that potently suppresses IgE-mediated type I hypersensitivity reactions. Structural analysis reveals that the purified anti-allergic compound (Perilla-derived methoxyflavanone, PDMF) is identified as 8-Hydroxy-5,7-dimethoxyflavanone. PDMF significantly inhibits IgE-mediated histamine release from RBL-2H3 rat basophilic leukemia cells as compared with those seen in known P. frutescens-derived anti-inflammatory polyphenols. We also show that oral administration of PDMF not only suppresses passive cutaneous anaphylaxis, but also prevents allergic rhinitis-like nasal symptoms in a murine model of Japanese cedar pollinosis. Mechanistically, PDMF negatively regulates Akt phosphorylation and intracellular Ca(2+) influx, both of which are essential for mast cell secretory granule translocation and its exocytosis upon high-affinity IgE receptor (FcepsilonRI) cross-linking. These results represent PDMF as a new potent anti-allergic phytochemical useful for prevention of IgE-driven hypersensitivity reactions.


