Ephedrannin ACAS# 82001-39-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 82001-39-6 | SDF | Download SDF |
| PubChem ID | 21676348 | Appearance | Powder |
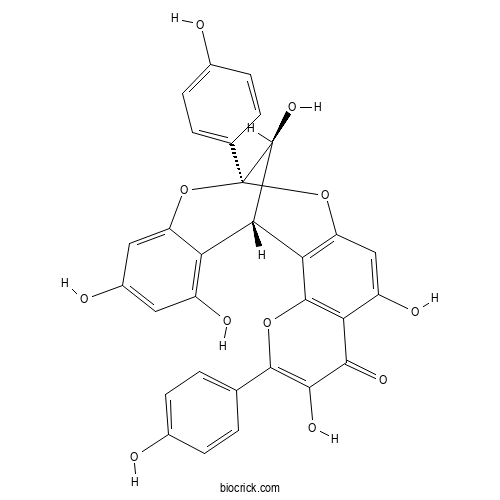
| Formula | C30H20O11 | M.Wt | 556.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1S,13R,21S)-6,9,17,19,21-pentahydroxy-5,13-bis(4-hydroxyphenyl)-4,12,14-trioxapentacyclo[11.7.1.02,11.03,8.015,20]henicosa-2(11),3(8),5,9,15,17,19-heptaen-7-one | ||
| SMILES | C1=CC(=CC=C1C2=C(C(=O)C3=C(O2)C4=C(C=C3O)OC5(C(C4C6=C(C=C(C=C6O5)O)O)O)C7=CC=C(C=C7)O)O)O | ||
| Standard InChIKey | GPBSBBVDERLESN-QZFRTWIZSA-N | ||
| Standard InChI | InChI=1S/C30H20O11/c31-14-5-1-12(2-6-14)27-26(37)25(36)22-18(35)11-20-23(28(22)39-27)24-21-17(34)9-16(33)10-19(21)40-30(41-20,29(24)38)13-3-7-15(32)8-4-13/h1-11,24,29,31-35,37-38H/t24-,29-,30+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ephedrannin A Dilution Calculator

Ephedrannin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7969 mL | 8.9847 mL | 17.9695 mL | 35.9389 mL | 44.9236 mL |
| 5 mM | 0.3594 mL | 1.7969 mL | 3.5939 mL | 7.1878 mL | 8.9847 mL |
| 10 mM | 0.1797 mL | 0.8985 mL | 1.7969 mL | 3.5939 mL | 4.4924 mL |
| 50 mM | 0.0359 mL | 0.1797 mL | 0.3594 mL | 0.7188 mL | 0.8985 mL |
| 100 mM | 0.018 mL | 0.0898 mL | 0.1797 mL | 0.3594 mL | 0.4492 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ganoderic acid GS-1
Catalog No.:BCX0057
CAS No.:1206781-64-7
- Yakuchinone A
Catalog No.:BCX0056
CAS No.:78954-23-1
- Tectorigenin 7-O-gentiobioside
Catalog No.:BCX0055
CAS No.:67604-94-8
- Peltatoside 7-O-beta-glucopyranoside
Catalog No.:BCX0054
CAS No.:813466-12-5
- Protocatechuic acid 4-O-beta-glucoside
Catalog No.:BCX0053
CAS No.:7361-59-3
- Methyl nomilinate
Catalog No.:BCX0052
CAS No.:77887-51-5
- 1-Phenylethyl beta-D-glucoside
Catalog No.:BCX0051
CAS No.:93199-03-2
- Ganoderiol D
Catalog No.:BCX0050
CAS No.:114567-45-2
- Geissoschizine
Catalog No.:BCX0049
CAS No.:439-66-7
- Taxezopidine H
Catalog No.:BCX0048
CAS No.:205440-23-9
- Calleryanin
Catalog No.:BCX0047
CAS No.:20300-53-2
- Mahuannin B
Catalog No.:BCX0046
CAS No.:82796-37-0
- Nomilinic acid
Catalog No.:BCX0059
CAS No.:35930-20-2
- Oxyphyllol B
Catalog No.:BCX0060
CAS No.:226546-99-2
- Ganosinensic acid C
Catalog No.:BCX0061
CAS No.:2231756-23-1
- 8-Hydroxy-5,7-dimethoxyflavanone
Catalog No.:BCX0062
CAS No.:201230-40-2
- Methyl ganoderate E
Catalog No.:BCX0063
CAS No.:98718-43-5
- Methyl ganoderate F
Catalog No.:BCX0064
CAS No.:98665-08-8
- Schisphenlignan I
Catalog No.:BCX0065
CAS No.:1542234-14-9
- Methyl ganoderate D
Catalog No.:BCX0066
CAS No.:97210-12-3
- Mahuannin E
Catalog No.:BCX0067
CAS No.:1173887-70-1
- Resinacein D
Catalog No.:BCX0068
CAS No.:2231061-47-3
- 1-O-Feruloylglucose
Catalog No.:BCX0069
CAS No.:64625-37-2
- 11alpha-hydroxy-3,7-dioxo-5alpha-lanosta-8,24(E)-dien-26-oic acid
Catalog No.:BCX0070
CAS No.:1245703-70-1
Inhibitory effect of ephedrannins A and B from roots of Ephedra sinica STAPF on melanogenesis.[Pubmed:25857772]
Biochim Biophys Acta. 2015 Jul;1850(7):1389-96.
BACKGROUND: Melanogenesis, a process producing the pigment melanin in human skin, eyes and hair, is a major physiological response against various environmental stresses, in particular exposure to ultraviolet radiation, and its pathway is regulated by a key enzyme, tyrosinase. In this study, we evaluated the effects of ephedrannins A and B, which are polyphenols from the roots of Ephedra sinica, commonly used in herbalism in oriental countries, on mushroom tyrosinase and melanogenesis in B16F10 melanoma cells. METHODS: Their effects on mushroom tyrosinase were determined via kinetic studies using a spectrophotometric analysis and those on melanin and tyrosinase production in melanoma cells treated with alpha-MSH (melanin stimulating hormone) were examined using PCR and ELISA. RESULTS: Both ephedrannins A and B exhibited concentration-dependent inhibitory effects on L-tyrosine oxidation by mushroom tyrosinase, and the inhibition mechanism was competitive and reversible with L-tyrosine as the substrate. In addition, melanin production in melanoma cells was also suppressed in a concentration-dependent manner by ephedrannins A and B without significant effects on cell proliferation at the concentrations tested. Both compounds showed inhibitory effects on melanin production by suppressing the transcription of tyrosinase in the cells. CONCLUSION: Both compounds exhibited significant inhibitory effects, but the inhibition by ephedrannin B was much more effective than that by Ephedrannin A. Both ephedrannins A and B may be good candidates for a whitening agent for skin. GENERAL SIGNIFICANCE: This is the first report that describes effective inhibition of melanin production by ephedrannins A and B isolated from Ephedra roots.
Ephedrannin A and B from roots of Ephedra sinica inhibit lipopolysaccharide-induced inflammatory mediators by suppressing nuclear factor-kappaB activation in RAW 264.7 macrophages.[Pubmed:20939997]
Int Immunopharmacol. 2010 Dec;10(12):1616-25.
Ephedra sinica is a traditional Chinese medicinal herb and has pharmacological functions including anti-inflammatory effects. However, the active ingredients from Ephedra roots have not been characterized. Here, two active constituents were isolated and their structures and mechanisms of action were defined. Active constituents from Ephedra roots were isolated by continuous solvent-extractions and column chromatography. Their structures were determined by use of multiple types of spectrometry. The mechanisms of action were examined using lipopolysaccharide (LPS)-stimulated RAW 264.7 cells through PCR, ELISA, electrophoretic mobility shift assays, and immunocytochemistry. Two active constituents, Ephedrannin A and B, belonging to the A-type proanthocyanidin family were identified. Both Ephedrannin A and B effectively suppressed the transcription of tumor necrosis factor-alpha (TNF-alpha) and interleukin-1beta (IL-1beta). These compounds exerted their anti-inflammatory actions on LPS-stimulated macrophages by suppressing the translocation of nuclear factor-kappa B (NF-kappaB) and the phosphorylation of p38 mitogen-activated protein (MAP) kinase. Ephedrannin A and B both exhibited strong anti-inflammatory effects, however, the optimal dose of ephedrannin B was 10 times lower than that of Ephedrannin A. This is the first report describing effective anti-inflammatory activity for Ephedrannin A and B isolated from Ephedra roots. Ephedrannin B may be a good candidate for delaying the progression of human inflammatory diseases and warrants further studies.
Dimeric proanthocyanidins from the roots of Ephedra sinica.[Pubmed:18975262]
Planta Med. 2008 Dec;74(15):1823-5.
Two new dimeric proanthocyanidins, ephedrannin B ( 2) and mahuannin E ( 4), along with two known congeners, Ephedrannin A ( 1) and mahuannin D ( 3), were isolated from the roots of Ephedra sinica Stapf. Based on NMR, MS, and CD analysis, the structures of the new compounds were deduced to be 5,7,4'-trihydroxyflavan-[4(alpha)-->8,2(alpha)-->O-->7]-kaempferol ( 2) and 5,7,4'-trihydroxyflavan-[4(beta)-->8,2(beta)-->O-->7]- EPI-afzelechin ( 4). The compounds 1 - 4 were evaluated for cytotoxicity against three tumor cell lines (SGC-7901, HepG2, and HeLa), and 2 was found to be significantly active.


