Yakuchinone ACAS# 78954-23-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 78954-23-1 | SDF | File under preparation. |
| PubChem ID | 133145 | Appearance | Powder |
| Formula | C20H24O3 | M.Wt | 312.4 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
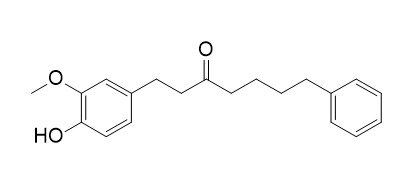
| Chemical Name | 1-(4-hydroxy-3-methoxyphenyl)-7-phenylheptan-3-one | ||
| SMILES | COC1=C(C=CC(=C1)CCC(=O)CCCCC2=CC=CC=C2)O | ||
| Standard InChIKey | TXELARZTKDBEKS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H24O3/c1-23-20-15-17(12-14-19(20)22)11-13-18(21)10-6-5-9-16-7-3-2-4-8-16/h2-4,7-8,12,14-15,22H,5-6,9-11,13H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Yakuchinone A Dilution Calculator

Yakuchinone A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.201 mL | 16.0051 mL | 32.0102 mL | 64.0205 mL | 80.0256 mL |
| 5 mM | 0.6402 mL | 3.201 mL | 6.402 mL | 12.8041 mL | 16.0051 mL |
| 10 mM | 0.3201 mL | 1.6005 mL | 3.201 mL | 6.402 mL | 8.0026 mL |
| 50 mM | 0.064 mL | 0.3201 mL | 0.6402 mL | 1.2804 mL | 1.6005 mL |
| 100 mM | 0.032 mL | 0.1601 mL | 0.3201 mL | 0.6402 mL | 0.8003 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tectorigenin 7-O-gentiobioside
Catalog No.:BCX0055
CAS No.:67604-94-8
- Peltatoside 7-O-beta-glucopyranoside
Catalog No.:BCX0054
CAS No.:813466-12-5
- Protocatechuic acid 4-O-beta-glucoside
Catalog No.:BCX0053
CAS No.:7361-59-3
- Methyl nomilinate
Catalog No.:BCX0052
CAS No.:77887-51-5
- 1-Phenylethyl beta-D-glucoside
Catalog No.:BCX0051
CAS No.:93199-03-2
- Ganoderiol D
Catalog No.:BCX0050
CAS No.:114567-45-2
- Geissoschizine
Catalog No.:BCX0049
CAS No.:439-66-7
- Taxezopidine H
Catalog No.:BCX0048
CAS No.:205440-23-9
- Calleryanin
Catalog No.:BCX0047
CAS No.:20300-53-2
- Mahuannin B
Catalog No.:BCX0046
CAS No.:82796-37-0
- Symplocoside
Catalog No.:BCX0045
CAS No.:76502-76-6
- Oblongaroside B
Catalog No.:BCX0044
CAS No.:1000889-11-1
- Ganoderic acid GS-1
Catalog No.:BCX0057
CAS No.:1206781-64-7
- Ephedrannin A
Catalog No.:BCX0058
CAS No.:82001-39-6
- Nomilinic acid
Catalog No.:BCX0059
CAS No.:35930-20-2
- Oxyphyllol B
Catalog No.:BCX0060
CAS No.:226546-99-2
- Ganosinensic acid C
Catalog No.:BCX0061
CAS No.:2231756-23-1
- 8-Hydroxy-5,7-dimethoxyflavanone
Catalog No.:BCX0062
CAS No.:201230-40-2
- Methyl ganoderate E
Catalog No.:BCX0063
CAS No.:98718-43-5
- Methyl ganoderate F
Catalog No.:BCX0064
CAS No.:98665-08-8
- Schisphenlignan I
Catalog No.:BCX0065
CAS No.:1542234-14-9
- Methyl ganoderate D
Catalog No.:BCX0066
CAS No.:97210-12-3
- Mahuannin E
Catalog No.:BCX0067
CAS No.:1173887-70-1
- Resinacein D
Catalog No.:BCX0068
CAS No.:2231061-47-3
Protective Effect of Alpinia oxyphylla Fruit against tert-Butyl Hydroperoxide-Induced Toxicity in HepG2 Cells via Nrf2 Activation and Free Radical Scavenging and Its Active Molecules.[Pubmed:35624896]
Antioxidants (Basel). 2022 May 23;11(5):1032.
Alpinia oxyphylla Miq. (Zingiberaceae) extract exerts protective activity against tert-butyl hydroperoxide-induced toxicity in HepG2 cells, and the antioxidant response element (ARE) luciferase activity increased 6-fold at 30 mug/mL in HepG2 cells transiently transfected with ARE-luciferase. To identify active molecules, activity-guided isolation of the crude extract led to four sesquiterpenes (1, 2, 5, 6) and two diarylheptanoids (3 and 4) from an n-hexane extract and six sesquiterpenes (7-12) from an ethyl acetate extract. Chemical structures were elucidated by one-dimensional, two-dimensional nuclear magnetic resonance (1D-, 2D-NMR), and mass (MS) spectral data. Among the isolated compounds, eudesma-3,11-dien-2-one (2) promoted the nuclear accumulation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and increased the promoter property of the ARE. Diarylheptanoids, Yakuchinone A (3), and 5'-hydroxyl-Yakuchinone A (4) showed radical scavenging activity in 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 3-ethylbenzothiazoline-6-sulphonic acid (ABTS) assays. Furthermore, optimization of extraction solvents (ratios of water and ethanol) was performed by comparison of contents of active compounds, ARE-inducing activity, radical scavenging activity, and HepG2 cell protective activity. As a result, 75% ethanol was the best solvent for the extraction of A. oxyphylla fruit. This study demonstrated that A. oxyphylla exerted antioxidant effects via the Nrf2/HO-1 (heme oxygenase-1) pathway and radical scavenging along with active markers eudesma-3,11-dien-2-one (2) and Yakuchinone A (3).
Microbial Transformation of Yakuchinone A and Cytotoxicity Evaluation of Its Metabolites.[Pubmed:35409351]
Int J Mol Sci. 2022 Apr 3;23(7):3992.
Yakuchinone A (1) is a bioactive diarylheptanoid isolated from the dried fruits of Alpinia oxyphylla. Microbial transformation has been recognized as an efficient method to produce new biologically active derivatives from natural products. In the present study, microbial transformation of Yakuchinone A was performed with the fungus Mucor hiemalis KCTC 26779, which led to the isolation of nine new metabolites (2, 3a, 3b, and 4-9). Their structures were elucidated as (3S)-oxyphyllacinol (2), (3S,7R)- and (3S,7S)-7-hydroxyoxyphyllacinol (3a and 3b), (3S)-oxyphyllacinol-4'-O-beta-d-glucopyranoside (4), (3S)-4''-hydroxyoxyphyllacinol (5), (3S)-3''-hydroxyoxyphyllacinol (6), (3S)-2''-hydroxyoxyphyllacinol (7), (3S)-2''-hydroxyoxyphyllacinol-2''-O-beta-d-glucopyranoside (8), and (3S)-oxyphyllacinol-3-O-beta-d-glucopyranoside (9) based on the comprehensive spectroscopic analyses and the application of modified Mosher's method. All compounds were evaluated for their cytotoxic activities against melanoma, as well as breast, lung, and colorectal cancer cell lines. Compound 9, which was O-glucosylated on the diarylheptanoid alkyl chain, exhibited the most selective cytotoxic activities against melanoma cell lines with the IC(50) values ranging from 6.09 to 9.74 muM, indicating that it might be considered as a possible anti-cancer lead compound.
Curcumin analogs as the inhibitors of TLR4 pathway in inflammation and their drug like potentialities: a computer-based study.[Pubmed:32223496]
J Recept Signal Transduct Res. 2020 Aug;40(4):324-338.
Toll-like receptor 4 (TLR4) pathway is one of the major pathways that mediate the inflammation in human body. There are different anti-inflammatory drugs available in the market which specifically act on different signaling proteins of TLR4 pathway but they do have few side effects and other limitations for intended use in human body. In this study, Curcumin and its different analogs have been analyzed as the inhibitors of signaling proteins, i.e. Cycloxygenase-2 (COX-2), inhibitor of kappabeta kinase (IKK) and TANK binding kinase-1 (TBK-1) of TLR4 pathway using different computational tools. Initially, three compounds were selected for respective target based on free binding energy among which different compounds were reported to have better binding affinity than commercially available drug (control). Upon continuous computational exploration with induced fit docking (IFD), 6-Gingerol, Yakuchinone A and Yakuchinone B were identified as the best inhibitors of COX-2, IKK, and TBK-1 respectively. Then their drug-like potentialities were analyzed in different experiments where they were also predicted to perform well. Hopefully, this study will uphold the efforts of researchers to identify anti-inflammatory drugs from natural sources.
Alpinia oxyphylla Fruit Extract Ameliorates Experimental Autoimmune Encephalomyelitis through the Regulation of Th1/Th17 Cells.[Pubmed:31001353]
Evid Based Complement Alternat Med. 2019 Mar 14;2019:6797030.
Alpinia oxyphylla is a traditional Chinese medicine widely used for treating diarrhea, ulceration, and enuresis. Moreover, A. oxyphylla is effective for cognitive function improvement and nerve regeneration. Multiple sclerosis (MS) is a chronic neuronal inflammatory autoimmune disease that commonly affects young adults in high-latitude regions. The aim of this study was to evaluate the beneficial effects of A. oxyphylla in an experimental autoimmune encephalomyelitis (EAE) mouse model, which is an extensively used model for human MS. The ethanolic extract of A. oxyphylla fruit (AO-1) was orally administered to EAE mice. Our results showed AO-1 significantly reduced EAE symptoms. Histopathological analysis showed AO-1 reduced demyelination, inflammation, gliosis, and axonal swelling in the spinal cord. Furthermore, immunohistochemistry and quantitative polymerase chain reaction (qPCR) studies revealed that the infiltration of CD4(+), CD8(+) T cells, and CD11b(+) monocytes into the spinal cord decreased in the AO-1-treated group. Mechanistically, the Th1 transcription factor T-bet, Th17 transcription factor retinoic acid receptor-related orphan receptor gamma (RORgammat), and inflammatory cytokines interferon (IFN)-gamma and interleukin (IL)-17 were reduced in the spinal cords of mice treated with AO-1. The expression levels of T-bet and RORgammat were also lowered in the spleens of those mice. Further in vitro study showed AO-1 inhibited production of IFN-gamma, IL-2, and tumor necrosis factor-alpha from MOG(35-55)-peptide-stimulated splenocytes. One component isolated from AO-1, Yakuchinone A, inhibited IL-17 production in vitro and reduced EAE symptoms in the mice. Collectively, our results indicate that AO-1 ameliorated the severity of EAE in mice and may involve the regulation of Th1/Th17 response. A. oxyphylla warrants further investigation, particularly regarding its clinical benefits for MS.
Development and validation of an LC-MS/MS method for the determination of DPHB in rat plasma and its application in pharmacokinetic studies.[Pubmed:30133708]
Biomed Chromatogr. 2018 Dec;32(12):e4373.
The aim of the present study was to develop a rapid, specific and sensitive LC-MS/MS method for the determination of DPHB [7-(4''-hydroxy-3''-methoxyphenyl)-1-phenyl-4-hepten-3-one] in rat plasma using Yakuchinone A as an internal standard (IS). n-Hexane was used for the extraction of DPHB from rat plasma. Chromatographic separation of DPHB was achieved using a Kinetex XB-C(18) column (2.10 x 50 mm, 2.6 mum) at 40 degrees C. The mobile phase consisted of water (containing 0.1 per thousand formic acid, A) and acetonitrile (containing 0.1 per thousand formic acid, B) under a gradient elution at a flow rate of 0.3 mL min(-1) . Positive electrospray ionization and multiple reaction monitoring mode were used for detection. The selected precursor ion to product ion pairs, m/z 311.3 --> 137.0 for DPHB and m/z 313.1 --> 137.0 for Yakuchinone A, were monitored. Good linearity was observed over the concentration range from 2 to 2000 ng mL(-1) (r = 0.9969). The recovery efficiency of DPHB from rat plasma was 54.8-69.7%, while the matrix effect ranged from 99.7 to 113%. Intra- and inter-day precision and accuracy values were within +/-15% at three different quality control concentration levels. This validated method was successfully applied to pharmacokinetic studies in rats after a single p.o. or i.v. dose of DPHB solution. The route of administration significantly influenced systemic exposure to DPHB, and low bioavailability of DPHB was observed. The method developed here will be further improved and used in future pharmacokinetic studies.
Izalpinin from fruits of Alpinia oxyphylla with antagonistic activity against the rat bladder contractility.[Pubmed:25392590]
Afr J Tradit Complement Altern Med. 2014 Jun 4;11(4):120-5.
BACKGROUND: Alpinia oxyphylla (Zingiberaceae), an herbaceous perennial plant, its capsular fruit is commonly used in traditional Chinese medicine for the treatment of different urinary incontinence symptoms including frequency, urgency and nocturia. These symptoms are similar to the overactive bladder syndrome. In our lab, we found that the 95% ethanol extract of the capsular fruits exhibited significant anti-muscarinic activity. Some constituents in capsular fruits including flavonoids (e.g., izalpinin and tectochrysin), diarylheptanoids (e.g., Yakuchinone A and yakuchinone B) and sesquiterpenes (e.g., nootkatone), are regarded as representative chemicals with putative pharmacological activities. OBJECTIVE: This study aimed to evaluate the in vitro antagonistic actions of izalpinin on carbachol-induced contraction of the rat detrusor muscle. MATERIALS AND METHODS: In vitro inhibition of rat detrusor contractile response to carbachol was used to study the functional activity of izalpinin. The isolated detrusor strips of rats were mounted in organ baths containing oxygenated Krebs' solution. The cumulative consecutive concentration-response curves to carbachol-evoked contractions in strips of rat bladder were obtained. RESULTS: Carbachol induced concentration-dependent contractions of isolated rat bladder detrusor strips. The vehicle DMSO had no impact on the contraction response. The contraction effects were concentration-dependently antagonized by izalpinin, with a mean EC50 value of 0.35 microM. The corresponding cumulative agonist concentration-response curves shifted right-ward. CONCLUSIONS: Izalpinin exhibits inhibitory role of muscarinic receptor-related detrusor contractile activity, and it may be a promising lead compound to treat overactive bladder.
Identification of known chemicals and their metabolites from Alpinia oxyphylla fruit extract in rat plasma using liquid chromatography/tandem mass spectrometry (LC-MS/MS) with selected reaction monitoring.[Pubmed:24879483]
J Pharm Biomed Anal. 2014 Aug;97:166-77.
Alpinia oxyphylla (Yizhi) capsularfruits are commonly used in traditional medicine. Pharmacological studies have demonstrated that A. oxyphylla capsularfruits have some beneficial roles. Besides volatile oil, sesquiterpenes, diarylheptanoids and flavonoids are main bioactive constituents occurring in the Yizhi capsularfruits. The representative constituents include tectochrysin, izalpinin, chrysin, apigenin-4',7-dimethylether, kaempferide, Yakuchinone A, yakuchinone B, oxyphyllacinol and nootkatone. Their content levels in the fruit and its pharmaceutical preparations have been reported by our group. The nine phytochemicals are also the major components present in the Yizhi alcoholic extracts, which have anti-diarrheal activities. However, the fates of these constituents in the body after oral or intravenous administration remain largely unknown. In the present study, we focus on these phytochemicals albeit other concomitant compounds. The chemicals and their metabolites in rat plasma were identified using liquid chromatography/tandem mass spectrometry with selected reaction monitoring mode after orally administered Yizhi extract to rats. Rat plasma samples were treated by methanol precipitation, acidic or enzymatic hydrolysis. This target analysis study revealed that: (1) low or trace plasma levels of parent chemicals were measured after p.o. administration of Yizhi extract, Suoquan capsules and pills to rats; (2) flavonoids and diarylheptanoids formed mainly monoglucuronide metabolites; however, diglucuronide metabolites for chrysin, izalpinin and kaempferide were also detected; (3) metabolic reduction of Yizhi diarylheptanoids occurred in rats. Yakuchinone B was reduced to Yakuchinone A and then to oxyphyllacinol in a stepwise manner and subsequently glucuronidated by UDP-glucuronosyl transferase. Further research is needed to characterize the UDP-glucuronosyl transferase and reductase involved in the biotransformation of Yizhi chemicals.
Different accumulation profiles of multiple components between pericarp and seed of Alpinia oxyphylla capsular fruit as determined by UFLC-MS/MS.[Pubmed:24727421]
Molecules. 2014 Apr 10;19(4):4510-23.
Plant secondary metabolites are known to not only play a key role in the adaptation of plants to their environment, but also represent an important source of active pharmaceuticals. Alpinia oxyphylla capsular fruits, made up of seeds and pericarps, are commonly used in traditional East Asian medicines. In clinical utilization of these capsular fruits, inconsistent processing approaches (i.e., hulling pericarps or not) are employed, with the potential of leading to differential pharmacological effects. Therefore, an important question arises whether the content levels of pharmacologically active chemicals between the seeds and pericarps of A. oxyphylla are comparable. Nine secondary metabolites present in A. oxyphylla capsular fruits, including flavonoids (e.g., tectochrysin, izalpinin, chrysin, apigenin-4',7-dimethylether and kaempferide), diarylheptanoids (e.g., Yakuchinone A and B and oxyphyllacinol) and sesquiterpenes (e.g., nootkatone), were regarded as representative constituents with putative pharmacological activities. This work aimed to investigate the abundance of the nine constituents in the seeds and pericarps of A. oxyphylla. Thirteen batches of A. oxyphylla capsular fruits were gathered from different production regions. Accordingly, an ultra-fast high performance liquid chromatography/quadrupole tandem mass spectrometry (UFLC-MS/MS) method was developed and validated. We found that: (1) the nine secondary metabolites were differentially concentrated in seeds and fruit capsules; (2) nootkatone is predominantly distributed in the seeds; in contrast, the flavonoids and diarylheptanoids are mainly deposited in the capsules; and (3) the content levels of the nine secondary metabolites occurring in the capsules varied greatly among different production regions, although the nootkatone levels in the seeds were comparable among production regions. These results are helpful to evaluating and elucidating pharmacological activities of A. oxyphylla capsular fruits. Additionally, it may be of interest to elucidate the mechanisms involved in the distinct accumulation profiles of these secondary metabolites between seeds and pericarps.
Validated method to measure yakuchinone A in plasma by LC-MS/MS and its application to a pharmacokinetic study in rats.[Pubmed:24422995]
Chem Cent J. 2014 Jan 14;8(1):2.
BACKGROUND: Yakuchinone A has a plethora of beneficial biological effects. However, the pharmacokinetic (PK) data of Yakuchinone A still remain unknown so far. Furthermore, the quantification of Yakuchinone A in biological samples has not been reported in the literature. Therefore, in the present study we aimed to develop a new method for the fast, efficient and accurate assessment of Yakuchinone A concentration in plasma, as a means for facilitating the PK evaluation of Yakuchinone A. RESULTS: A liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) method was developed and validated for the determination of Yakuchinone A in rat plasma. Mass spectrometric and chromatographic conditions were optimized. Plasma samples were pretreated by protein precipitation with methanol. LC separation was performed on a Phenomenex Luna C18 column with gradient elution using a mobile phase consisting of methanol-water containing 0.5 mM formic acid (HCOOH) at a flow rate of 0.28 mL/min. ESI-MS spectra were acquired in positive ion multiple reaction monitoring mode (MRM). The precursor-to-product ion pairs used for MRM of Yakuchinone A and yakuchinone B were m/z 313.1 --> 137.0 and 311.2 --> 117.1, respectively. Low concentration of HCOOH reduced the ion suppression caused by matrix components and clearly improved the analytical sensitivity. Yakuchinone A showed good linearity over a wide concentration range (r > 0.99). The accuracy, precision, stability and linearity were found to be within the acceptable criteria. This new method was successfully applied to analyze the rat plasma concentration of parent Yakuchinone A after a single oral administration of SuoQuan capsules. Low systemic exposure to parent Yakuchinone A was observed. CONCLUSION: The proposed method is sensitive and reliable. It is hoped that this new method will prove useful for the future PK studies.
Antioxidant, anti-adipocyte differentiation, antitumor activity and anthelmintic activities against Anisakis simplex and Hymenolepis nana of yakuchinone A from Alpinia oxyphylla.[Pubmed:24070160]
BMC Complement Altern Med. 2013 Sep 26;13:237.
BACKGROUND: Alpinia oxyphylla is a common remedy in traditional Chinese medicine. Yakuchinone A is a major constituent of A. oxyphylla and exhibits anti-inflammatory, antitumor, antibacterial, and gastric protective activities. METHODS: Antioxidant and antitumor characteristics of Yakuchinone A in skin cancer cells as well as novel mechanisms for the inhibition of adipocyte differentiation, cestocidal activities against Hymenolepis nana adults, and nematocidal activities against Anisakis simplex larvae are investigated. RESULTS: Yakuchinone A presents the ability of the removal of DPPH.and ABTS+ free radicals and inhibition of lipid peroxidation. Yakuchinone A suppresses intracellular lipid accumulation during adipocyte differentiation in 3 T3-L1 cells and the expressions of leptin and peroxisome proliferator-activated receptor gamma (PPARgamma). Yakuchinone A induces apoptosis and inhibits cell proliferation in skin cancer cells. The inhibition of cell growth by Yakuchinone A is more significant for non-melanoma skin cancer (NMSC) cells than for melanoma (A375 and B16) and noncancerous (HaCaT and BNLCL2) cells. Treatment BCC cells with Yakuchinone A shows down-regulation of Bcl-2, up-regulation of Bax, and an increase in cleavage poly (ADP-ribose) polymerase (PARP). This suggests that Yakuchinone A induces BCC cells apoptosis through the Bcl-2-mediated signaling pathway. The anthelmintic activities of Yakuchinone A for A. simplex are better than for H. nana. CONCLUSIONS: In this work, Yakuchinone A exhibits antioxidative properties, anti-adipocyte differentiation, antitumor activity, and anthelmintic activities against A. simplex and H. nana.
Quantitative analysis of the major constituents in Chinese medicinal preparation SuoQuan formulae by ultra fast high performance liquid chromatography/quadrupole tandem mass spectrometry.[Pubmed:23899222]
Chem Cent J. 2013 Jul 30;7(1):131.
BACKGROUND: The SuoQuan formulae containing Fructus Alpiniae Oxyphyllae has been used to combat the urinary incontinence symptoms including frequency, urgency and nocturia for hundreds of years in China. However, the chemical information was not well characterized. The quality control marker constituent only focused on one single compound in the current Chinese Pharmacopeia. Hence it is prudent to identify and quantify the main constituents in this herbal product. This study aimed to analyze the main constituents using ultra-fast performance liquid chromatography coupled to tandem mass spectrometry (UFLC-MS/MS). RESULTS: Fourteen phytochemicals originated from five chemical classes constituents were identified by comparing the molecular mass, fragmentation pattern and retention time with those of the reference standards. A newly developed UFLC-MS/MS was validated demonstrating that the new assay was valid, reproducible and reliable. This method was successfully applied to simultaneously quantify the fourteen phytochemicals. Notably, the content of these constituents showed significant differences in three pharmaceutical preparations. The major constituent originated from each of chemical class was isolinderalactone, norisoboldine, nootkatone, Yakuchinone A and apigenin-4',7-dimethylther, respectively. The variation among these compounds was more than 1000 times. Furthermore, the significant content variation between the two different Suoquan pills was also observed. CONCLUSION: The proposed method is sensitive and reliable; hence it can be used to analyze a variety of SuoQuan formulae products produced by different pharmaceutical manufacturers.
Two new antioxidant diarylheptanoids from the fruits of Alpinia oxyphylla.[Pubmed:23869536]
J Asian Nat Prod Res. 2013;15(10):1094-9.
Two new diarylheptanoids, 1-(3',5'-dihydroxy-4'-methoxyphenyl)-7-phenyl-3-heptanone (1) and 1-(2',4'-dihydroxy-3'-methoxyphenyl)-7-(4''-methoxyphenyl)-3-heptanone (2), along with known diarylheptanoid Yakuchinone A (3), and five flavanoids, tectochrysin (4), chrysin (5), izalpinin (6), kaempferol 7, 4'-dimethyl ether (7), and kaempferide (8) were isolated from the fruits of Alpinia oxyphylla Miq. Their structures were determined by means of spectroscopic methods. Antioxidant activities of all the isolated compounds were evaluated using a 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay. Compounds 1-3 and 6-8 exhibited potent antioxidant activities in the DPPH assay.


