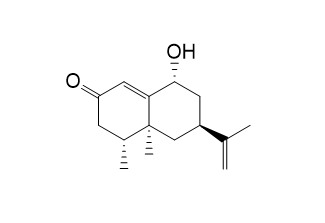
Oxyphyllol BCAS# 226546-99-2 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 226546-99-2 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Oil |
| Formula | C15H22O2 | M.Wt | 234.3 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Oxyphyllol B Dilution Calculator

Oxyphyllol B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.268 mL | 21.3402 mL | 42.6803 mL | 85.3606 mL | 106.7008 mL |
| 5 mM | 0.8536 mL | 4.268 mL | 8.5361 mL | 17.0721 mL | 21.3402 mL |
| 10 mM | 0.4268 mL | 2.134 mL | 4.268 mL | 8.5361 mL | 10.6701 mL |
| 50 mM | 0.0854 mL | 0.4268 mL | 0.8536 mL | 1.7072 mL | 2.134 mL |
| 100 mM | 0.0427 mL | 0.2134 mL | 0.4268 mL | 0.8536 mL | 1.067 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Nomilinic acid
Catalog No.:BCX0059
CAS No.:35930-20-2
- Ephedrannin A
Catalog No.:BCX0058
CAS No.:82001-39-6
- Ganoderic acid GS-1
Catalog No.:BCX0057
CAS No.:1206781-64-7
- Yakuchinone A
Catalog No.:BCX0056
CAS No.:78954-23-1
- Tectorigenin 7-O-gentiobioside
Catalog No.:BCX0055
CAS No.:67604-94-8
- Peltatoside 7-O-beta-glucopyranoside
Catalog No.:BCX0054
CAS No.:813466-12-5
- Protocatechuic acid 4-O-beta-glucoside
Catalog No.:BCX0053
CAS No.:7361-59-3
- Methyl nomilinate
Catalog No.:BCX0052
CAS No.:77887-51-5
- 1-Phenylethyl beta-D-glucoside
Catalog No.:BCX0051
CAS No.:93199-03-2
- Ganoderiol D
Catalog No.:BCX0050
CAS No.:114567-45-2
- Geissoschizine
Catalog No.:BCX0049
CAS No.:439-66-7
- Taxezopidine H
Catalog No.:BCX0048
CAS No.:205440-23-9
- Ganosinensic acid C
Catalog No.:BCX0061
CAS No.:2231756-23-1
- 8-Hydroxy-5,7-dimethoxyflavanone
Catalog No.:BCX0062
CAS No.:201230-40-2
- Methyl ganoderate E
Catalog No.:BCX0063
CAS No.:98718-43-5
- Methyl ganoderate F
Catalog No.:BCX0064
CAS No.:98665-08-8
- Schisphenlignan I
Catalog No.:BCX0065
CAS No.:1542234-14-9
- Methyl ganoderate D
Catalog No.:BCX0066
CAS No.:97210-12-3
- Mahuannin E
Catalog No.:BCX0067
CAS No.:1173887-70-1
- Resinacein D
Catalog No.:BCX0068
CAS No.:2231061-47-3
- 1-O-Feruloylglucose
Catalog No.:BCX0069
CAS No.:64625-37-2
- 11alpha-hydroxy-3,7-dioxo-5alpha-lanosta-8,24(E)-dien-26-oic acid
Catalog No.:BCX0070
CAS No.:1245703-70-1
- Clinopodic acid E
Catalog No.:BCX0071
CAS No.:159736-38-6
- N-Methyltetrahydrocolumbamine
Catalog No.:BCX0072
CAS No.:92758-34-4
DIURETIC AND ANTI-DIURETIC BIOACTIVITY DIFFERENCES OF THE SEED AND SHELL EXTRACTS OF ALPINIA OXYPHYLLA FRUIT.[Pubmed:28487890]
Afr J Tradit Complement Altern Med. 2016 Aug 12;13(5):25-32.
BACKGROUND: Alpinia oxyphylla fruit (AOF, Yizhi in Chinese) is a well-known traditional Chinese medicine as an anti-diuretic agent and composed of two parts i.e. seed and shell. These two parts have different components, but the bioactivity differences of the two parts are not clear. This study aims to evaluate the different anti-diuretic effects of the seed and shell of AOF. MATERIALS AND METHODS: The potential bioactive components were analyzed by UPLC-Q-TOF-MS. The diuretic and anti-diuretic activity was determined with saline-loads rats. RESULTS: The results showed that the 200 mg/kg and 400mg/kg of SREAO displayed a short-time anti-diuretic activity 1h after administration and then a significant diuretic activity was being observed at 5-6 h in 400mg/kg group of SREAO. And the 400mg/kg doses of SREAO also showed a remarkable increase for electrolyte excretion of K(+). Three sesquiterpene compounds, namely oxyphyllol A (1), Oxyphyllol B (2), and nootkatone (3) were identified from the active SREAO fraction by UHPLC-ESI-Q-TOF/MS. CONCLUSION: The seed part of Alpinia oxyphylla possessed pronounced diuretic and anti-diuretic effect. The sesquiterpene components are the major constituents and possibly contributed the diuretic and anti-diuretic activity.
Collective total synthesis of englerin A and B, orientalol E and F, and oxyphyllol: application of the organocatalytic [4+3] cycloaddition reaction.[Pubmed:23292997]
Chemistry. 2013 Feb 11;19(7):2539-47.
The concise collective total synthesis of englerin A and B, orientalol E and F, and oxyphyllol has been accomplished in 10-15 steps, with the total synthesis of orientalol E and Oxyphyllol Being achieved for the first time. The success obtained was enabled by the realization of the [4+3] cycloaddition reaction of 9 and 10. Other features of the synthesis include 1) the intramolecular Heck reaction to access the azulene core, 2) the epoxidation-S(N)2' reduction sequence to access the allylic alcohol, 3) the efficient regioselective and stereoselective formal hydration of the bridging C=C bond in the synthesis of englerins, and 4) the late-stage chemo- and stereoselective C-H oxidation in the synthesis of orientalol E. The total synthesis of these natural products has enabled the structural revision of oxyphyllol and established the absolute stereochemical features of the organocatalytic [4+3] cycloaddition reaction. The identification of 5 as the natural product oxyphyllol, the success in converting 5 to orientalol E, along with the fact that englerins and oxyphyllol were isolated from plants of the same genus Phyllanthus gives support to our proposed biosynthetic pathways. This work may enable detailed biological evaluations of these natural products and their analogues and derivatives, especially of their potential in the fight against renal cell carcinoma (RCC).


