8-Hydroxy-PIPAT oxalateHigh affinity 5-HT1A agonist CAS# 1451210-48-2 |
- PI 828
Catalog No.:BCC7494
CAS No.:942289-87-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1451210-48-2 | SDF | Download SDF |
| PubChem ID | 56972162 | Appearance | Powder |
| Formula | C18H24INO5 | M.Wt | 461.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in ethanol and to 100 mM in DMSO | ||
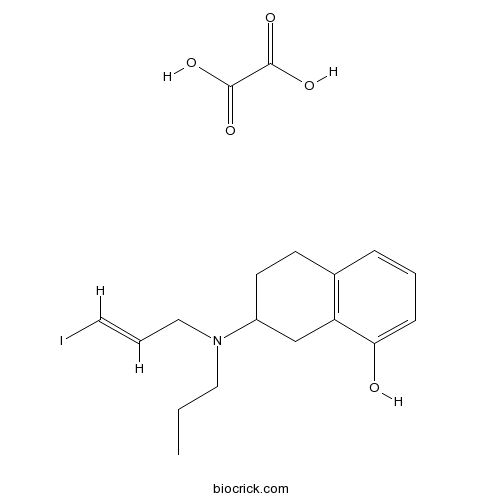
| Chemical Name | 7-[[(E)-3-iodoprop-2-enyl]-propylamino]-5,6,7,8-tetrahydronaphthalen-1-ol;oxalic acid | ||
| SMILES | CCCN(CC=CI)C1CCC2=C(C1)C(=CC=C2)O.C(=O)(C(=O)O)O | ||
| Standard InChIKey | XIBSIFOFYWBOAQ-JOKMOOFLSA-N | ||
| Standard InChI | InChI=1S/C16H22INO.C2H2O4/c1-2-10-18(11-4-9-17)14-8-7-13-5-3-6-16(19)15(13)12-14;3-1(4)2(5)6/h3-6,9,14,19H,2,7-8,10-12H2,1H3;(H,3,4)(H,5,6)/b9-4+; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | High affinity 5-HT1A receptor agonist (Kd = 0.38 nM). 7-Hydroxy-PIPAT maleate also available. |

8-Hydroxy-PIPAT oxalate Dilution Calculator

8-Hydroxy-PIPAT oxalate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1678 mL | 10.8389 mL | 21.6779 mL | 43.3557 mL | 54.1947 mL |
| 5 mM | 0.4336 mL | 2.1678 mL | 4.3356 mL | 8.6711 mL | 10.8389 mL |
| 10 mM | 0.2168 mL | 1.0839 mL | 2.1678 mL | 4.3356 mL | 5.4195 mL |
| 50 mM | 0.0434 mL | 0.2168 mL | 0.4336 mL | 0.8671 mL | 1.0839 mL |
| 100 mM | 0.0217 mL | 0.1084 mL | 0.2168 mL | 0.4336 mL | 0.5419 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dexmedetomidine HCl
Catalog No.:BCC4347
CAS No.:145108-58-3
- Piribedil dihydrochloride
Catalog No.:BCC6898
CAS No.:1451048-94-4
- Filicenol B
Catalog No.:BCN6446
CAS No.:145103-37-3
- YM 022
Catalog No.:BCC7052
CAS No.:145084-28-2
- Rink Amide Linker
Catalog No.:BCC2833
CAS No.:145069-56-3
- CPI-169
Catalog No.:BCC5396
CAS No.:1450655-76-1
- Candesartan cilexetil
Catalog No.:BCC8900
CAS No.:145040-37-5
- FGIN-1-43
Catalog No.:BCC6739
CAS No.:145040-29-5
- Fmoc-Asp(OMe)-OH
Catalog No.:BCC3090
CAS No.:145038-53-5
- Digalactosyldiacylglycerol
Catalog No.:BCC8941
CAS No.:145033-48-3
- ω-Agatoxin IVA
Catalog No.:BCC7488
CAS No.:145017-83-0
- Pregnenolone
Catalog No.:BCN6255
CAS No.:145-13-1
- 6-Hydroxykaempferol-3,6,7-triglucoside
Catalog No.:BCN1562
CAS No.:145134-62-9
- 6-Hydroxykaempferol 3-Rutinoside -6-glucoside
Catalog No.:BCN1561
CAS No.:145134-63-0
- CP 99994 dihydrochloride
Catalog No.:BCC6016
CAS No.:145148-39-6
- 3beta-Hydroxyporiferast-5-en-7-one
Catalog No.:BCN6256
CAS No.:145163-97-9
- Iodophenpropit dihydrobromide
Catalog No.:BCC6793
CAS No.:145196-87-8
- Kaliotoxin
Catalog No.:BCC7710
CAS No.:145199-73-1
- Rizatriptan Benzoate
Catalog No.:BCC3852
CAS No.:145202-66-0
- Punctanecine
Catalog No.:BCN2018
CAS No.:145204-91-7
- CALP1
Catalog No.:BCC5873
CAS No.:145224-99-3
- Clobenpropit dihydrobromide
Catalog No.:BCC6781
CAS No.:145231-35-2
- Delgrandine
Catalog No.:BCN8122
CAS No.:145237-05-4
- Sahandol
Catalog No.:BCN6996
CAS No.:1452398-07-0
Calcium oxalate crystal related kidney injury in a patient receiving Roux-en Y hepaticojejunostomy due to gall bladder cancer.[Pubmed:28356078]
BMC Nephrol. 2017 Mar 29;18(1):106.
BACKGROUND: Calcium oxalate nephropathy is rare in current practice. It was a common complication during jejunoileal bypass, but much less seen in modern gastric bypass surgery for morbid obesity. The major cause of it is enteric hyperoxaluria. CASE PRESENTATION: We report on a patient here with acute kidney disease due to calcium oxalate nephropathy, rather than the conditions mentioned above. The male patient received a Roux-en Y hepaticojejunostomy and common bile duct drainage. In addition to enteric hyperoxaluria, chronic kidney disease related metabolic acidosis, chronic diarrhea related volume depletion, a high oxalate and low potassium diet, long term ascorbic acid intake and long term exposure to antibiotics, all predisposed him to having oxalate nephropathy. CONCLUSION: This is the first case with such conditions and we recommend that similarly diagnosed patients avoid all these predisposing factors, in order to avoid this rare disease and its undesired outcome.
Pathogenesis of calcium oxalate urinary stone disease: species comparison of humans, dogs, and cats.[Pubmed:28361470]
Urolithiasis. 2017 Aug;45(4):329-336.
Idiopathic calcium oxalate nephrolithiasis is a highly recurrent disease that is increasing in prevalence. Decades of research have not identified effective methods to consistently prevent the formation of nephroliths or induce medical dissolution. Idiopathic calcium oxalate nephroliths form in association with renal papillary subepithelial calcium phosphate deposits called Randall's plaques (RPs). Rodent models are commonly used to experimentally induce calcium oxalate crystal and stone formation, but a rodent model that conclusively forms RPs has not been identified. Both dogs and cats form calcium oxalate uroliths that can be recurrent, but the etiopathologic mechanisms of stone formation, especially renal pathologic findings, are a relatively unexploited area of study. A large animal model that shares a similar environment to humans, along with a shorter lifespan and thus shorter time to recurrence, might provide an excellent means to study preventative and therapeutic measures, along with enhancing the concepts of the one health initiative. This review article summarizes and compares important known features of idiopathic calcium oxalate stone disease in humans, dogs, and cats, and emphasizes important knowledge gaps and areas for future study in the quest to discover a naturally occurring animal model of idiopathic calcium oxalate stone disease.
[Expression of matrix Gla protein and bone morphogenetic protein 2 in renal papillary tissues in patients with calcium oxalate kidney stones].[Pubmed:28364100]
Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2017 Mar 28;42(3):277-283.
OBJECTIVE: To compare expression levels of matrix Gla protein (MGP) and bone morphogenetic protein 2 (BMP-2) in Randall's plaque of renal papillary tissues in patients with calcium oxalate kidney stones and the underlying mechanism for stone formation. Methods: A total of 30 samples of Randall's plaque in renal papillary tissues from patients with calcium oxalate kidney stones were collected from the Department of Urology of Xiangya Hospital of Central South University from April, 2015 to December, 2015 and served as an experimental group. Ten samples of renal papillary tissues in patients undergone renal tumor nephrectomy were collected from the same hospital and served as a control group. The expressions of MGP and BMP-2 mRNA and protein were detected by quantitative real-time PCR and Western blot.Meanwhile, immunohistochemical technique was used to observe the expressions of MGP and BMP-2 in different parts of renal papillary tissues in the 2 groups. Results: 1) The mRNA expression levels of MGP in the experimental group and the control group were 0.760+/-0.804 and 1.365+/-0.348, respectively, with significant difference between them (P<0.05). Them RNA levels of BMP-2 in the experimental group and the control group were 2.500+/-0.725 and 1.485+/-0.870, respectively, with significant difference between them (P<0.05). The expression levels of MGP protein in the experimental group and the control group were 0.130+/-0.424 and 0.202+/-0.704, respectively, with no significant difference between them (P>0.05). The expression levels of BMP-2 protein in the experimental group and the control group were 0.885+/-0.220 and 0.682+/-0.272, respectively, with significant difference between them (P<0.05). The immunohistochemistry showed that the protein expression of MGP in the experimental group was lower than that in the control group, while the protein expression of BMP-2 in the experimental group was higher than that in the control group (both P<0.05). Conclusion: The BMP-2 expression is increased while MGP expression is decreased in renal papillary tissues in patients with calcium oxalate kidney stones, and the formation of calcium oxalate kidney stone might be a kind of osteogenetic reaction or ectopic calcification.
The influence of citrate and oxalate on (99)Tc(VII), Cs, Np(V) and U(VI) sorption to a Savannah River Site soil.[Pubmed:28351009]
J Environ Radioact. 2017 Jun;172:130-142.
Batch sorption experiments were conducted with 0.5-50 ppb (99)Tc, (133)Cs, (237)Np and U in the presence and absence of citrate and/or oxalate in a 25 g/L Savannah River Site (SRS) soil suspension. Citrate and oxalate were the ligands of choice due to their relevancy to plant exudates, the nuclides were selected for their wide range of biogeochemical behavior, and the soil from SRS was selected as a model Department of Energy (DOE) site soil. Batch samples were continually mixed on a rotary shaker and maintained at a pH of approximately 5. Analysis via ICP-MS indicated that sorption of (237)Np increased with ligand concentration compared to baseline studies, as did sorption of (99)Tc although to a lesser extent. The increased sorption of (237)Np is proposed to be due to a combination of factors that are dependent on the ligand(s) present in the specific system including, ligand dissolution of the soil by citrate and formation of tertiary soil-oxalate-Np complexes. The increased (99)Tc sorption is attributed to the dissolution of the soil by the ligands, leading to an increase in the number of available sorption sites for (99)Tc. Uranium sorption decreased and dissolution of native uranium was also observed with increasing ligand concentration, thought to be a result of the formation of strong U-ligand complexes remaining in the aqueous phase. The majority of these effects were observed at the highest ligand concentrations of 50 mgC/L. No notable changes were observed for the (133)Cs system which is ascribed to the minimal interaction of Cs(+) with these organic ligands.
Synthesis of (R,S)-trans-8-hydroxy-2-[N-n-propyl-N-(3'-iodo-2'-propenyl)amino]tetral in (trans-8-OH-PIPAT): a new 5-HT1A receptor ligand.[Pubmed:8230102]
J Med Chem. 1993 Oct 15;36(21):3161-5.
In order to develop tracers with higher specific activity to supplant the currently used [3H]-8-OH-DPAT [8-hydroxy-2-(N,N-di-n-propylamino)tetralin] for in vitro and in vivo evaluation of 5-HT1A receptors, a new radioiodinated ligand was prepared. (R,S)-trans-8- Hydroxy-2-[N-n-propyl-N-(3'-iodo-2'-propenyl)amino]tetralin (trans-8-OH-PIPAT), 8, was synthesized by a 10-step reaction. Binding studies with rat hippocampal membrane homogenates showed that 8 exhibited a Ki value of 0.92 nM against (R,S)-[3H]-8-OH-DPAT. Radiolabeled [125I]-8 was prepared from the corresponding tri-n-butyltin precursor via an oxidative iododestannylation reaction with sodium [125I]iodide. Binding studies in the hippocampal homogenates revealed that [125I]-8 bound to a single high-affinity site (Kd = 0.38 +/- 0.03 nM,Bmax = 310 +/- 20 fmol/mg of protein). Competition binding experiments clearly indicated that the new ligand displayed the expected 5-HT1A receptor binding profile. The rank order of potency was (R,S)-trans-8-OH-PIPAT > (R,S)- 8-OH-DPAT > WB4101 > 5-HT > (R,S)-trans-7-OH-PIPAT > (R,S)-7-OH-DPAT > (R,S)-propranolol > spiperone >> ketanserin >> dopamine > atropine. This new ligand offers several unique advantages, including high specific activity, high binding affinity, and low nonspecific binding, all of which make it an excellent probe for the investigation and characterization of 5-HT1A receptors.


