Rink Amide LinkerCAS# 145069-56-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 145069-56-3 | SDF | Download SDF |
| PubChem ID | 2761459 | Appearance | Powder |
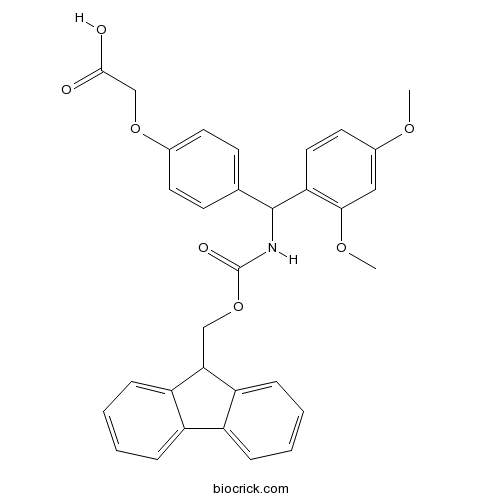
| Formula | C32H29NO7 | M.Wt | 539.6 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-[4-[(2,4-dimethoxyphenyl)-(9H-fluoren-9-ylmethoxycarbonylamino)methyl]phenoxy]acetic acid | ||
| SMILES | COC1=CC(=C(C=C1)C(C2=CC=C(C=C2)OCC(=O)O)NC(=O)OCC3C4=CC=CC=C4C5=CC=CC=C35)OC | ||
| Standard InChIKey | UPMGJEMWPQOACJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C32H29NO7/c1-37-22-15-16-27(29(17-22)38-2)31(20-11-13-21(14-12-20)39-19-30(34)35)33-32(36)40-18-28-25-9-5-3-7-23(25)24-8-4-6-10-26(24)28/h3-17,28,31H,18-19H2,1-2H3,(H,33,36)(H,34,35) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Rink Amide Linker Dilution Calculator

Rink Amide Linker Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8532 mL | 9.2661 mL | 18.5322 mL | 37.0645 mL | 46.3306 mL |
| 5 mM | 0.3706 mL | 1.8532 mL | 3.7064 mL | 7.4129 mL | 9.2661 mL |
| 10 mM | 0.1853 mL | 0.9266 mL | 1.8532 mL | 3.7064 mL | 4.6331 mL |
| 50 mM | 0.0371 mL | 0.1853 mL | 0.3706 mL | 0.7413 mL | 0.9266 mL |
| 100 mM | 0.0185 mL | 0.0927 mL | 0.1853 mL | 0.3706 mL | 0.4633 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Rink Amide Linker
- CPI-169
Catalog No.:BCC5396
CAS No.:1450655-76-1
- Candesartan cilexetil
Catalog No.:BCC8900
CAS No.:145040-37-5
- FGIN-1-43
Catalog No.:BCC6739
CAS No.:145040-29-5
- Fmoc-Asp(OMe)-OH
Catalog No.:BCC3090
CAS No.:145038-53-5
- Digalactosyldiacylglycerol
Catalog No.:BCC8941
CAS No.:145033-48-3
- ω-Agatoxin IVA
Catalog No.:BCC7488
CAS No.:145017-83-0
- Pregnenolone
Catalog No.:BCN6255
CAS No.:145-13-1
- DGAT1-IN-1
Catalog No.:BCC5511
CAS No.:1449779-49-0
- DDR1-IN-1
Catalog No.:BCC5170
CAS No.:1449685-96-4
- Boeravinone O
Catalog No.:BCN6693
CAS No.:1449384-21-7
- Chlojaponilactone B
Catalog No.:BCN7400
CAS No.:1449382-91-5
- K145 hydrochloride
Catalog No.:BCC4072
CAS No.:1449240-68-9
- YM 022
Catalog No.:BCC7052
CAS No.:145084-28-2
- Filicenol B
Catalog No.:BCN6446
CAS No.:145103-37-3
- Piribedil dihydrochloride
Catalog No.:BCC6898
CAS No.:1451048-94-4
- Dexmedetomidine HCl
Catalog No.:BCC4347
CAS No.:145108-58-3
- 8-Hydroxy-PIPAT oxalate
Catalog No.:BCC6800
CAS No.:1451210-48-2
- 6-Hydroxykaempferol-3,6,7-triglucoside
Catalog No.:BCN1562
CAS No.:145134-62-9
- 6-Hydroxykaempferol 3-Rutinoside -6-glucoside
Catalog No.:BCN1561
CAS No.:145134-63-0
- CP 99994 dihydrochloride
Catalog No.:BCC6016
CAS No.:145148-39-6
- 3beta-Hydroxyporiferast-5-en-7-one
Catalog No.:BCN6256
CAS No.:145163-97-9
- Iodophenpropit dihydrobromide
Catalog No.:BCC6793
CAS No.:145196-87-8
- Kaliotoxin
Catalog No.:BCC7710
CAS No.:145199-73-1
- Rizatriptan Benzoate
Catalog No.:BCC3852
CAS No.:145202-66-0
C-terminal N-alkylated peptide amides resulting from the linker decomposition of the Rink amide resin: a new cleavage mixture prevents their formation.[Pubmed:16103992]
J Pept Sci. 2006 Mar;12(3):227-32.
Decomposition of the resin linkers during TFA cleavage of the peptides in the Fmoc strategy leads to alkylation of sensitive amino acids. The C-terminal amide alkylation, reported for the first time, is shown to be a major problem in peptide amides synthesized on the Rink amide resin. This side reaction occurs as a result of the Rink Amide Linker decomposition under TFA treatment of the peptide resin. The use of 1,3-dimethoxybenzene in a cleavage cocktail prevents almost quantitatively formation of C-terminal N-alkylated peptide amides. Oxidized by-product in the tested Cys- and Met-containing peptides were not observed, even if thiols were not used in the cleavage mixture.
Solid phase chemical ligation employing a rink amide linker for the synthesis of histone H2B protein.[Pubmed:25196573]
Chem Commun (Camb). 2014 Oct 25;50(83):12534-7.
Presented here is a solid phase chemical ligation strategy employing native chemical ligation and the commercially available Rink-amide linker as a key element in our approach. The method was applied for the synthesis of histone H2B, which sets the ground for the rapid preparation of posttranslationally modified analogues of this protein.


