ATPACAS# 140158-50-5 |
- Pitavastatin
Catalog No.:BCC4140
CAS No.:147511-69-1
- Mevastatin
Catalog No.:BCN2568
CAS No.:73573-88-3
- Lovastatin
Catalog No.:BCN1060
CAS No.:75330-75-5
- Pravastatin
Catalog No.:BCC4141
CAS No.:81093-37-0
- Pravastatin sodium
Catalog No.:BCC2321
CAS No.:81131-70-6
- Fluvastatin Sodium
Catalog No.:BCC2317
CAS No.:93957-55-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 140158-50-5 | SDF | Download SDF |
| PubChem ID | 2253 | Appearance | Powder |
| Formula | C10H16N2O4 | M.Wt | 228.25 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in water and to 100 mM in 1eq. NaOH | ||
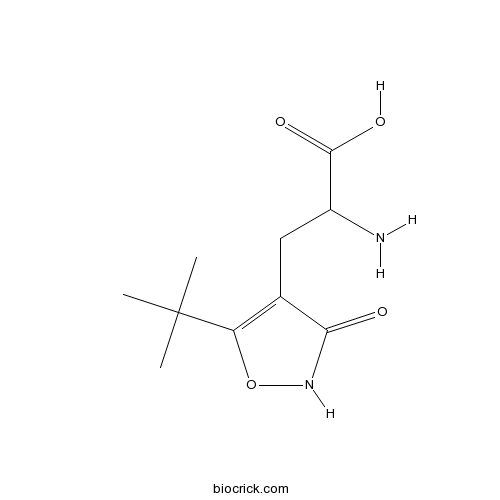
| Chemical Name | 2-amino-3-(5-tert-butyl-3-oxo-1,2-oxazol-4-yl)propanoic acid | ||
| SMILES | CC(C)(C)C1=C(C(=O)NO1)CC(C(=O)O)N | ||
| Standard InChIKey | PIXJURSCCVBKRF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H16N2O4/c1-10(2,3)7-5(8(13)12-16-7)4-6(11)9(14)15/h6H,4,11H2,1-3H3,(H,12,13)(H,14,15) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A selective and potent GluR5 kainate receptor agonist (Ki = 4.3 nM), inactive at GluR6 (Ki > 1 mM) and only weakly active at AMPA receptors (GluR1-4) and the kainate receptors KA-2 and GluR7 (Ki values of 6 - 14 μM). Also available as part of the Kainate Receptor. |

ATPA Dilution Calculator

ATPA Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3812 mL | 21.9058 mL | 43.8116 mL | 87.6232 mL | 109.529 mL |
| 5 mM | 0.8762 mL | 4.3812 mL | 8.7623 mL | 17.5246 mL | 21.9058 mL |
| 10 mM | 0.4381 mL | 2.1906 mL | 4.3812 mL | 8.7623 mL | 10.9529 mL |
| 50 mM | 0.0876 mL | 0.4381 mL | 0.8762 mL | 1.7525 mL | 2.1906 mL |
| 100 mM | 0.0438 mL | 0.2191 mL | 0.4381 mL | 0.8762 mL | 1.0953 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Epimedin A1
Catalog No.:BCN5935
CAS No.:140147-77-9
- Pterisolic acid F
Catalog No.:BCN4840
CAS No.:1401419-90-6
- Pterisolic acid E
Catalog No.:BCN4841
CAS No.:1401419-89-3
- Pterisolic acid D
Catalog No.:BCN4839
CAS No.:1401419-88-2
- Pterisolic acid C
Catalog No.:BCN4838
CAS No.:1401419-87-1
- Pterisolic acid B
Catalog No.:BCN4843
CAS No.:1401419-86-0
- Pterisolic acid A
Catalog No.:BCN4842
CAS No.:1401419-85-9
- 15,16-Dihydroxyoctadeca-9Z,12Z-dienoic acid
Catalog No.:BCC8438
CAS No.:140129-22-2
- ML 277
Catalog No.:BCC7976
CAS No.:1401242-74-7
- PI-1840
Catalog No.:BCC5453
CAS No.:1401223-22-0
- 3'-Hydroxygynuramide II
Catalog No.:BCC8634
CAS No.:1401093-57-9
- AMG 925
Catalog No.:BCC5150
CAS No.:1401033-86-0
- Negundonorin A
Catalog No.:BCN7150
CAS No.:1401618-51-6
- (-)-Praeruptorin A
Catalog No.:BCN7664
CAS No.:14017-71-1
- Monnieriside G
Catalog No.:BCN7857
CAS No.:1401799-34-5
- Monnieriside A
Catalog No.:BCN7892
CAS No.:1401807-73-5
- (S)-(-)-5-Fluorowillardiine
Catalog No.:BCC6596
CAS No.:140187-23-1
- (S)-(-)-5-Iodowillardiine
Catalog No.:BCC6597
CAS No.:140187-25-3
- Mc-MMAD
Catalog No.:BCC1735
CAS No.:1401963-15-2
- Vc-MMAD
Catalog No.:BCC2032
CAS No.:1401963-17-4
- GSK2879552
Catalog No.:BCC6422
CAS No.:1401966-69-5
- Psiguadial D
Catalog No.:BCN7086
CAS No.:1402066-95-8
- Eupalinolide I
Catalog No.:BCN7367
CAS No.:1402067-84-8
- TUG-770
Catalog No.:BCC2018
CAS No.:1402601-82-4
Assessment of the role in protection and pathogenesis of the Chlamydia muridarum V-type ATP synthase subunit A (AtpA) (TC0582).[Pubmed:24161793]
Microbes Infect. 2014 Feb;16(2):123-133.
A novel Chlamydia muridarum antigen (TC0582) was used to vaccinate BALB/c mice. Mice were also immunized with other components of the ATP synthase complex (TC0580, TC0581, and TC0584), or with the major outer membrane protein (MOMP). TC0582 was also formulated in combination with TC0580, TC0581 or MOMP. TC0582 alone, or in combination with the other antigens, elicited strong Chlamydia-specific humoral and cellular immune responses. Vaccinated animals were challenged intranasally and the course of the infection was followed for 10 days. Based on percentage change in body weight, lung weight, and number of Chlamydia inclusion forming units recovered from the lungs, mice immunized with TC0582, TC0581 or MOMP, as single antigens, showed significant protection. Mice immunized with combinations of two antigens were also protected but the level of protection was not additive. TC0582 has sequence homology with the eukaryotic ATP synthase subunit A (ATPA). Therefore, to determine if immunization with TC0582, or with Chlamydia, elicited antibodies that cross-reacted with the mouse ATPA, the two proteins were printed on a microarray. Sera from mice immunized with TC0582 and/or live Chlamydia, strongly reacted with TC0582 but did not recognize the mouse ATPA. In conclusion, TC0582 may be considered as a Chlamydia vaccine candidate.
Use of an improved atpA amplification and sequencing method to identify members of the Campylobacteraceae and Helicobacteraceae.[Pubmed:24517729]
Lett Appl Microbiol. 2014 Jun;58(6):582-90.
UNLABELLED: Emerging Campylobacter and Arcobacter spp. have been increasingly isolated from human clinical samples, food, veterinary samples and the environment. Unambiguous species identification of such organisms is of obvious importance in epidemiological studies, but is also necessary to accurately assess their host range and determine their prevalence in the food chain and in the environment. Species identification methods for the Campylobacteraceae have been described; however, some with high resolving power are limited to a small number of taxa, while other broader-range methods cannot distinguish between closely related species. We present in this study a novel species identification method, based on amplification and sequencing of a portion of the ATPA gene. This method, which uses a single primer pair, was able to amplify and accurately identify all current taxa within Campylobacter and Arcobacter as well as several members of the Helicobacteraceae, although unambiguous identification of the Camp. fetus subspecies could not be achieved. In addition, five putative novel Campylobacter taxa were recognized, making this new species identification method valuable in the characterization of novel epsilonproteobacteria. Thus, a single-locus method that can accurately identify multiple epsilonproteobacterial species will prove important in the characterization of emerging organisms and those associated with illness. SIGNIFICANCE AND IMPACT OF THE STUDY: The ATPA-based species identification method described here uses a single primer pair to amplify DNA from all current validly-described Campylobacter and Arcobacter taxa, as well as multiple members of the Helicobacteraceae. This method unambiguously identified all taxa tested, although it could not discriminate the subspecies of Camp. fetus. Furthermore, five putative novel Campylobacter taxa were observed following testing of environmental campylobacters with this method. The scope and resolution of this method make it an important addition to studies of epsilonproteobacterial epidemiology and evolution.
ATPA induced GluR5-containing kainite receptor S-nitrosylation via activation of GluR5-Gq-PLC-IP(3)R pathway and signalling module GluR5.PSD-95.nNOS.[Pubmed:23000395]
Int J Biochem Cell Biol. 2012 Dec;44(12):2261-71.
GluR5-containing kainite receptor (GluR5-KAR) plays an important role in the pathophysiology of nervous system diseases, while S-nitrosylation exerts a variety of effects on biological systems. However, the mechanism of GluR5-KAR S-nitrosylation is still unclear up to now. Here our researches found that GluR5-KAR selective agonist ATPA stimulation activated the nonclassical GluR5-KAR-Gq-PLC-IP(3)R pathway and the signalling module GluR5.PSD-95.nNOS (the former is more important), led to Ca(2+) release from intracellular calcium stores endoplasmic reticulum (ER) to cytoplasm and extracellular calcium indrawal, respectively, which further resulted in nNOS activation and GluR5-KAR S-nitrosylation, and then inhibited GluR5-mediated whole-cell current attenuation and induced apoptosis in primary cultured hippocampal neurons. Clarification of the primary mechanisms of GluR5-KAR S-nitrosylation induced by ATPA and identification of critical cysteine for GluR5-2a S-nitrosylation (Cys231 and Cys804) open up a brand-new field for revealing downstream signalling pathway of GluR5-KAR and its molecular characteristics, exploring the pathogenesis of neurological diseases and searching for promising therapies.
Excitotoxic injury profiles of low-affinity kainate receptor agonists in cortical neuronal cultures.[Pubmed:10478637]
Eur J Pharmacol. 1999 Aug 6;378(2):R1-3.
Neurotoxic profiles of putative agonists for low-affinity kainate subtypes of L-glutamate receptors (GluR5-7) were determined in cultured cortical neurones. Rank order of neurotoxic potency (microM): (S)-5-iodowillardiine (9) approximately = (2S,4R,6E)-2-amino-4-carboxy-7-(2-naphthyl)hept-6-enoic acid (LY339434, 11) > (2S,4R)-4-methylglutamate (33) > kainate (100) > (RS)-2-amino-3-(hydroxy-5-tert-butylisoxazol-4-yl)propanoic acid (ATPA, 360). Using ionotropic glutamate receptor antagonists, neurotoxicity induced by kainate, ATPA and (S)-5-iodowillardiine appeared to involve a GluR5-7 component, unlike LY339434 and (2S,4R)-4-methylglutamate. These putative GluR5-7 agonists exhibited complex excitotoxic profiles highlighting the importance of studying native glutamate receptors.
A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission.[Pubmed:9335499]
Nature. 1997 Oct 9;389(6651):599-603.
The principal excitatory neurotransmitter in the vertebrate central nervous system, L-glutamate, acts on three classes of ionotripic glutamate receptors, named after the agonists AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxalole-4-propionic acid), NMDA (N-methyl-D-aspartate) and kainate. The development of selective pharmacological agents has led to a detailed understanding of the physiological and pathological roles of AMPA and NMDA receptors. In contrast, the lack of selective kainate receptor ligands has greatly hindered progress in understanding the roles of kainate receptors. Here we describe the effects of a potent and selective agonist, ATPA ((RS)-2-amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl)propanoic acid) and a selective antagonist, LY294486 ((3SR, 4aRS, 6SR, 8aRS)-6-((((1H-tetrazol-5-yl) methyl)oxy)methyl)-1, 2, 3, 4, 4a, 5, 6, 7, 8, 8a-decahydroisoquinoline-3-carboxylic acid), of the GluR5 subtype of kainate receptor. We have used these agents to show that kainate receptors, comprised of or containing GluR5 subunits, regulate synaptic inhibition in the hippocampus, an action that could contribute to the epileptogenic effects of kainate.
AMPA receptor agonists: synthesis, protolytic properties, and pharmacology of 3-isothiazolol bioisosteres of glutamic acid.[Pubmed:9046343]
J Med Chem. 1997 Feb 14;40(4):520-7.
A number of 3-isothiazolol bioisosteres of glutamic acid (1) and analogs of the AMPA receptor agonist, (RS)-2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl)propionic acid (AMPA, 2a), including (RS)-2-amino-3-(3-hydroxy-5-methylisothiazol-4-yl)propionic acid (thio-AMPA, 2b), were synthesized. Comparative in vitro pharmacological studies on this series of 3-isothiazolol and the corresponding 3-isoxazolol amino acids were performed using a series of receptor binding assays (IC50 values) and the electrophysiological rat cortical slice model (EC50 values). Whereas 2a (IC50 = 0.04 +/- 0.005 microM, EC50 = 3.5 +/- 0.2 microM) is markedly more potent than the tert-butyl analog ATPA (3a) (IC50 = 2.1 +/- 0.16 microM, EC50 = 34 +/- 2.4 microM) in [3H]AMPA binding and electrophysiological studies, 2b (IC50 = 1.8 +/- 0.13 microM, EC50 = 15.0 +/- 2.4 microM) was approximately equipotent with thio-ATPA (3b) (IC50 = 0.63 +/- 0.07 microM, EC50 = 14 +/- 1.3 microM). (RS)-2-Amino-3-(3-hydroxyisoxazol-5-yl)propionic acid (HIBO, 4a) was approximately equipotent with its thio analog 4b, whereas 4-Br-HIBO (5a) (IC50 = 0.65 +/- 0.12 microM, EC50 = 22 +/- 0.6 microM) turned out to be much more potent than the corresponding 3-isothiazolol 5b (IC50 = 17 +/- 2.2 microM, EC50 = 500 +/- 23 microM). 2b (ED50 = 130 mumol/kg) was more potent than 2a (220 mumol/kg) as a convulsant after subcutaneous administration in mice. The protolytic properties of 2a,b-4a,b were determined using 13C NMR spectroscopy. For each pair of compounds, the alpha-amino acid groups showed similar protolytic properties, whereas the 3-isoxazolol moieties typically showed pKa values 2 units lower than those of the 3-isothiazolols. Accordingly, calculations of ionic species distributions revealed pronounced differences between 3-isoxazolol and 3-isothiazolol amino acids. No simple correlation between activity as AMPA agonists in vitro and pKa values of these compounds was apparent. On the other hand, the relative potencies of AMPA (2a) and thio-AMPA (2b) in vitro and in vivo may reflect that these compounds predominantly penetrate the blood-brain barrier as net uncharged diprotonated ionic species.


