AaptamineCAS# 85547-22-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 85547-22-4 | SDF | Download SDF |
| PubChem ID | 122826 | Appearance | Powder |
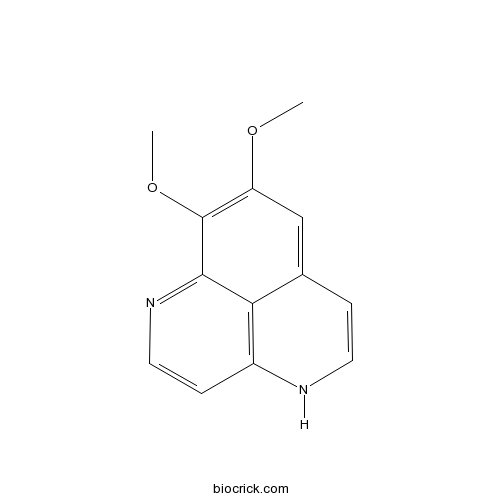
| Formula | C13H12N2O2 | M.Wt | 228.25 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | COC1=C(C2=NC=CC3=C2C(=C1)C=CN3)OC | ||
| Standard InChIKey | UERYGOYPBXIFQV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H12N2O2/c1-16-10-7-8-3-5-14-9-4-6-15-12(11(8)9)13(10)17-2/h3-7,14H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Aaptamine exerts an antiproliferative effect against NT2-R, a cisplatin-resistant subline of the human embryonal carcinoma cell line NT2. 2. Aaptamine activates p21 promoter in a p53-independent manner. 3. Aaptamine functions as a proteasome inhibitor. 4. Aaptamine is a competitive antagonist of alpha-adrenoceptors in vascular smooth muscles. 5. Aaptamine has potent cytotoxicity that may be explained by its ability to intercalate DNA. 6. Aaptamine shows anti-photoaging effect in UVB-irradiated human dermal fibroblasts and epidermal keratinocytes. 7. Aaptamine has anti-antiviral activity. |
| Targets | p53 | TNF-α | c-Myc | p21 | ROS | MAPK | COX | AP-1 | NF-kB | MMP(e.g.TIMP) | IL Receptor |

Aaptamine Dilution Calculator

Aaptamine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3812 mL | 21.9058 mL | 43.8116 mL | 87.6232 mL | 109.529 mL |
| 5 mM | 0.8762 mL | 4.3812 mL | 8.7623 mL | 17.5246 mL | 21.9058 mL |
| 10 mM | 0.4381 mL | 2.1906 mL | 4.3812 mL | 8.7623 mL | 10.9529 mL |
| 50 mM | 0.0876 mL | 0.4381 mL | 0.8762 mL | 1.7525 mL | 2.1906 mL |
| 100 mM | 0.0438 mL | 0.2191 mL | 0.4381 mL | 0.8762 mL | 1.0953 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Hydroquinidine
Catalog No.:BCN8589
CAS No.:1435-55-8
- Rubiayannone A
Catalog No.:BCN8588
CAS No.:517978-25-1
- Glyasperin C
Catalog No.:BCN8587
CAS No.:142474-53-1
- Sarracenin
Catalog No.:BCN8586
CAS No.:59653-37-1
- Platycodin D3
Catalog No.:BCN8585
CAS No.:67884-03-1
- Lucidin 3-O-primeveroside
Catalog No.:BCN8584
CAS No.:29706-59-0
- Ligupurpuroside C
Catalog No.:BCN8583
CAS No.:1194056-33-1
- Isorosmanol
Catalog No.:BCN8582
CAS No.:93780-80-4
- Isoginsenoside Rh3
Catalog No.:BCN8581
CAS No.:166040-90-0
- Uvarigrin
Catalog No.:BCN8580
CAS No.:200563-11-7
- 4-Benzamido-2,5-diethoxybenzenediazonium
Catalog No.:BCN8579
CAS No.:5486-84-0
- Hexamethylquercetagetin
Catalog No.:BCN8578
CAS No.:1251-84-9
- Firmanoic acid
Catalog No.:BCN8591
CAS No.:107584-83-8
- Licoflavone B
Catalog No.:BCN8592
CAS No.:91433-17-9
- Cinobufaginol
Catalog No.:BCN8593
CAS No.:6691-83-4
- Jasminoside B
Catalog No.:BCN8594
CAS No.:214125-04-9
- Ophiopogonoside A
Catalog No.:BCN8595
CAS No.:791849-22-4
- Dehydronuciferine
Catalog No.:BCN8596
CAS No.:7630-74-2
- Licoflavone A
Catalog No.:BCN8597
CAS No.:61153-77-3
- Eriosematin
Catalog No.:BCN8598
CAS No.:168010-17-1
- Isoastragaloside IV
Catalog No.:BCN8599
CAS No.:136033-55-1
- Astraganoside
Catalog No.:BCN8600
CAS No.:1011711-05-9
- Rubipodanone A
Catalog No.:BCN8601
CAS No.:2170211-22-8
- Asperosaponin IV
Catalog No.:BCN8602
CAS No.:126778-93-6
Activity of aaptamine and two derivatives, demethyloxyaaptamine and isoaaptamine, in cisplatin-resistant germ cell cancer.[Pubmed:24269226]
J Proteomics. 2014 Jan 16;96:223-39.
UNLABELLED: We analyzed the effects of all three marine alkaloids Aaptamine, demethyloxyAaptamine and isoAaptamine in NT2-R, a cisplatin-resistant subline of the human embryonal carcinoma cell line NT2. All Aaptamines were found to be equally effective in both cell lines, excluding cross-resistance between Aaptamines and cisplatin in vitro. At the inhibitory concentration (IC50), Aaptamine exerted an antiproliferative effect, whereas demethyloxyAaptamine and isoAaptamine were strong inducers of apoptosis. We analyzed the changes in the proteome of NT2-R cells treated with these compounds. 16-22 proteins were found to be significantly altered, of which several were validated by Western blotting and two-dimensional Western blotting analysis. Changes in the proteome pattern frequently resulted from post-transcriptional protein modifications, i.e. phosphorylation or hypusination in the case of eIF5A. Although the lists of altered proteins were heterogeneous and compound-specific, gene ontology analyses identified rather similar profiles regarding the affected molecular functions. Ingenuity pathway analysis by IPA put the following factors in a central position of the hypothetical networks: myc and p53 for Aaptamine; tumor necrosis factor (TNF) for demethyloxyAaptamine; and all three, myc, p53, and TNF for isoAaptamine. Our results represent an important step towards a better understanding of the molecular basis underlying the observed bioactivity of these promising marine compounds. BIOLOGICAL SIGNIFICANCE: We characterized the mode of action of three Aaptamines, marine natural compound with anti-tumor activity, using a functional proteomics approach and the cisplatin-resistant pluripotent human embryonal carcinoma cell line NT2-R. The manuscript is of particular scientific interest, as we could reveal the similarities and differences of the modes of action. Furthermore, we were able to identify several new targets of these promising compounds. We found hypusination of eIF5A to be a prominent feature exclusively of Aaptamine treatment, as this was not observed upon treatment with demethyloxyAaptamine or isoAaptamine. Our results are a step towards unraveling the mode of action of these interesting compounds.
Aaptamine, a spongean alkaloid, activates p21 promoter in a p53-independent manner.[Pubmed:16480688]
Biochem Biophys Res Commun. 2006 Mar 31;342(1):101-6.
Aaptamine, a benzonaphthyridine alkaloid was isolated from a marine sponge on the guidance of a bioassay using the transfected human osteosarcoma MG63 cells (MG63luc(+)). Aaptamine activated p21 promoter stably transfected in MG63 cells dose-dependently at the concentrations of 20-50microM. Expression of p21 and its mRNA in the wild-type MG63 cells also increased by Aaptamine-treatment. Furthermore, the cell cycle of MG63 cells was arrested at the G2/M phase within 48h by the Aaptamine-treatment. To analyze a responsive element of p21 promoter in the up-regulation of p21 by Aaptamine, MG63 cells were transiently transfected with a series of the deleted or mutated promoter segments, and induction of luciferase with Aaptamine treatment was examined by using these corresponding transfected cells. The activation of p21 promoter by Aaptamine was led through acting Sp1 sites between -82 and -50bp in a p53-independent manner.
Aaptamine, an alkaloid from the sponge Aaptos suberitoides, functions as a proteasome inhibitor.[Pubmed:20451377]
Bioorg Med Chem Lett. 2010 Jun 1;20(11):3341-3.
Aaptamine (1), isoAaptamine (2), and demethylAaptamine (3) were isolated from the marine sponge Aaptossuberitoides collected in Indonesia as inhibitors of the proteasome. They inhibited the chymotrypsin-like and caspase-like activities of the proteasome with IC(50) values of 1.6-4.6 microg/mL, while they showed less inhibition of the trypsin-like activity of the proteasome. The three compounds showed cytotoxic activities against HeLa cells, but their cytotoxicity did not correlate with their potency as proteasome inhibitors, strongly suggesting that their proteasomal inhibitory activity is dispensable to their cytotoxicity.
Anti-photoaging effect of aaptamine in UVB-irradiated human dermal fibroblasts and epidermal keratinocytes.[Pubmed:25465718]
J Asian Nat Prod Res. 2014 Dec;16(12):1139-47.
Chronic exposure to ultraviolet (UV) irradiation causes sunburn, inflammatory responses, skin cancer, and photoaging. Photoaging, in particular, generates reactive oxygen species (ROS) that stimulate mitogen-activated protein kinase (MAPK) signaling and transcription factors. UV irradiation also activates matrix metalloproteinases (MMPs) expression and inactivates collagen synthesis. Aaptamine, a marine alkaloid isolated from the marine sponge, has been reported to have antitumor, antimicrobial, antiviral, and antioxidant activities. However, the photo-protective effects of Aaptamine have not been elucidated. In this study, our data demonstrated that Aaptamine deactivated UVB-induced MAPK and activator protein-1 signaling by suppressing ROS, resulting in attenuating the expression of MMPs in UVB-irradiated human dermal fibroblasts. Aaptamine also decreased proinflammatory cytokines such as cyclooxygenase-2, tumor necrosis factor-alpha, interleukin-1beta, and nuclear factor-kappa B subunits in UVB-irradiated human keratinocytes. In conclusion, we suggest that Aaptamine represents a novel and effective strategy for treatment and prevention of photoaging.
Antiviral and anticancer optimization studies of the DNA-binding marine natural product aaptamine.[Pubmed:18251774]
Chem Biol Drug Des. 2008 Mar;71(3):205-15.
Aaptamine has potent cytotoxicity that may be explained by its ability to intercalate DNA. Aaptamine was evaluated for its ability to bind to DNA to validate DNA binding as the primary mechanism of cytotoxicity. Based on UV-vis absorbance titration data, the K(obs) for Aaptamine was 4.0 (+/-0.2) x 10(3) which was essentially equivalent to the known DNA intercalator N-[2-(diethylamino)ethyl]-9-aminoacridine-4-carboxamide. Semi-synthetic core modifications were performed to improve the general structural diversity of known Aaptamine analogs and vary its absorption characteristics. Overall, 26 Aaptamine derivatives were synthesized which consisted of a simple homologous range of mono and di-N-alkylations as well as some 9-O-sulfonylation and bis-O-isoAaptamine dimer products. Each product was evaluated for activity in a variety of whole cell and viral assays including a unique solid tumor disk diffusion assay. Details of Aaptamine's DNA-binding activity and its derivatives' whole cell and viral assay results are discussed.
Alpha-adrenoceptor blocking action of aaptamine, a novel marine natural product, in vascular smooth muscle.[Pubmed:6150989]
J Pharm Pharmacol. 1984 Nov;36(11):785-6.
In the rabbit isolated aorta and renal artery, Aaptamine (3 X 10(-5)M), a novel heteroaromatic substance isolated from a sea sponge Aaptos aaptos produced a parallel, rightward shift of the dose-response curve for noradrenaline, whereas that for histamine or KCl was not affected. But, the derivatives of Aaptamine, demethylAaptamine, demethyloxyAaptamine, dihydroAaptamine and dihydrodemethylAaptamine at concentrations of 10(-5) to 10(-4)M had no effect on the dose-response curve for noradrenaline. These results suggest that Aaptamine is a competitive antagonist of alpha-adrenoceptors in vascular smooth muscles.


