Licoflavone BCAS# 91433-17-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 91433-17-9 | SDF | Download SDF |
| PubChem ID | 11349817 | Appearance | Powder |
| Formula | C25H26O4 | M.Wt | 390.47 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
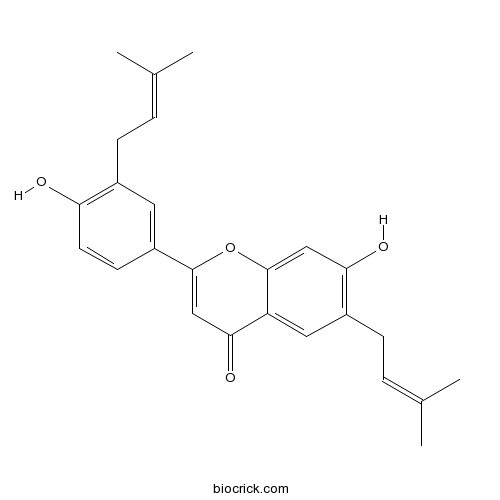
| Chemical Name | 7-hydroxy-2-[4-hydroxy-3-(3-methylbut-2-enyl)phenyl]-6-(3-methylbut-2-enyl)chromen-4-one | ||
| SMILES | CC(=CCC1=C(C=CC(=C1)C2=CC(=O)C3=CC(=C(C=C3O2)O)CC=C(C)C)O)C | ||
| Standard InChIKey | GLDVIKFETPAZNV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H26O4/c1-15(2)5-7-17-11-19(9-10-21(17)26)24-14-23(28)20-12-18(8-6-16(3)4)22(27)13-25(20)29-24/h5-6,9-14,26-27H,7-8H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Licoflavone B has schistosomicidal activity, it showed high S. mansoni ATPase (IC50 of 23.78 uM) and ADPase (IC50 of 31.50 uM) inhibitory activities. |
| Targets | Antifection | ATPase |

Licoflavone B Dilution Calculator

Licoflavone B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.561 mL | 12.8051 mL | 25.6102 mL | 51.2203 mL | 64.0254 mL |
| 5 mM | 0.5122 mL | 2.561 mL | 5.122 mL | 10.2441 mL | 12.8051 mL |
| 10 mM | 0.2561 mL | 1.2805 mL | 2.561 mL | 5.122 mL | 6.4025 mL |
| 50 mM | 0.0512 mL | 0.2561 mL | 0.5122 mL | 1.0244 mL | 1.2805 mL |
| 100 mM | 0.0256 mL | 0.1281 mL | 0.2561 mL | 0.5122 mL | 0.6403 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Firmanoic acid
Catalog No.:BCN8591
CAS No.:107584-83-8
- Aaptamine
Catalog No.:BCN8590
CAS No.:85547-22-4
- Hydroquinidine
Catalog No.:BCN8589
CAS No.:1435-55-8
- Rubiayannone A
Catalog No.:BCN8588
CAS No.:517978-25-1
- Glyasperin C
Catalog No.:BCN8587
CAS No.:142474-53-1
- Sarracenin
Catalog No.:BCN8586
CAS No.:59653-37-1
- Platycodin D3
Catalog No.:BCN8585
CAS No.:67884-03-1
- Lucidin 3-O-primeveroside
Catalog No.:BCN8584
CAS No.:29706-59-0
- Ligupurpuroside C
Catalog No.:BCN8583
CAS No.:1194056-33-1
- Isorosmanol
Catalog No.:BCN8582
CAS No.:93780-80-4
- Isoginsenoside Rh3
Catalog No.:BCN8581
CAS No.:166040-90-0
- Uvarigrin
Catalog No.:BCN8580
CAS No.:200563-11-7
- Cinobufaginol
Catalog No.:BCN8593
CAS No.:6691-83-4
- Jasminoside B
Catalog No.:BCN8594
CAS No.:214125-04-9
- Ophiopogonoside A
Catalog No.:BCN8595
CAS No.:791849-22-4
- Dehydronuciferine
Catalog No.:BCN8596
CAS No.:7630-74-2
- Licoflavone A
Catalog No.:BCN8597
CAS No.:61153-77-3
- Eriosematin
Catalog No.:BCN8598
CAS No.:168010-17-1
- Isoastragaloside IV
Catalog No.:BCN8599
CAS No.:136033-55-1
- Astraganoside
Catalog No.:BCN8600
CAS No.:1011711-05-9
- Rubipodanone A
Catalog No.:BCN8601
CAS No.:2170211-22-8
- Asperosaponin IV
Catalog No.:BCN8602
CAS No.:126778-93-6
- 9-Methymyrrhone
Catalog No.:BCN8603
CAS No.:1809980-22-0
- Uvarigranol C
Catalog No.:BCN8604
CAS No.:172104-04-0
Schistosomicidal activity and docking of Schistosoma mansoni ATPDase 1 with licoflavone B isolated from Glycyrrhiza inflata (Fabaceae).[Pubmed:26454044]
Exp Parasitol. 2015 Dec;159:207-14.
Schistosomiasis is one of the world's major public health problems, and its treatment is widely dependent on praziquantel (PZQ), the only available drug. Schistosoma mansoni ATP diphosphohydrolases are ecto-enzymes localized on the external tegumental surface of S. mansoni and considered an important target for action of new drugs. In this work, the in vitro schistosomicidal activity of the crude extract of Glycyrrhiza inflata roots (GI) and its isolated compounds echinatin, licoflavone A and Licoflavone B were evaluated against S. mansoni adult worms. Results showed that GI (200 mug/mL) was active against adult schistosomes, causing 100% mortality after 24 h of incubation. Chromatographic fractionation of GI led to isolation of echinatin, licoflavone A and Licoflavone B. Licoflavone B (25-100 muM) caused 100% mortality, tegumental alterations, and reduction of oviposition and motor activity of all adult worms, without affecting mammalian Vero cells. Confocal laser scanning microscopy showed tegumental morphological alterations and changes on the numbers of tubercles of S. mansoni worms in a dose-dependent manner after incubation with Licoflavone B. Licoflavone B also showed high S. mansoni ATPase (IC50 of 23.78 muM) and ADPase (IC50 of 31.50 muM) inhibitory activities. Docking studies predicted different interactions between Licoflavone B and S. mansoni ATPDase 1, corroborating with the in vitro inhibitory activity. This report demonstrated the first evidence for the schistosomicidal activity of Licoflavone B and suggests that its mechanism of action involve the inhibition of S. mansoni ATP diphosphohydrolases.
Computer-guided approach to access the anti-influenza activity of licorice constituents.[Pubmed:24313801]
J Nat Prod. 2014 Mar 28;77(3):563-70.
Neuraminidase (NA), a key enzyme in viral replication, is the first-line drug target to combat influenza. On the basis of a shape-focused virtual screening, the roots of Glycyrrhiza glabra (licorice) were identified as plant species with an accumulation of constituents that show 3D similarities to known influenza NA inhibitors (NAIs). Phytochemical investigation revealed 12 constituents identified as (E)-1-[2,4-dihydroxy-3-(3-methyl-2-butenyl)phenyl]-3-(8-hydroxy-2,2-dimethyl-2H-1 -benzopyran-6-yl)-2-propen-1-one (1), 3,4-dihydro-8,8-dimethyl-2H,8H-benzo[1,2-b:3,4-b']dipyran-3-ol (2), biochanin B (3), glabrol (4), glabrone (5), hispaglabridin B (6), Licoflavone B (7), licorice glycoside B (8), licorice glycoside E (9), liquiritigenin (10), liquiritin (11), and prunin (12). Eleven of these constituents showed significant influenza virus NA inhibition in a chemiluminescence (CL)-based assay. Additional tests, including (i) a cell-based cytopathic effect inhibition assay (general antiviral activity), (ii) the evaluation of cytotoxicity, (iii) the inhibition of the NA of Clostridium perfringens (CL- and fluorescence (FL)-based assay), and (iv) the determination of self-fluorescence and quenching, provided further perspective on their anti-influenza virus potential, revealing possible assay interference problems and false-positive results. Compounds 1, 3, 5, and 6 showed antiviral activity, most likely caused by the inhibition of NA. Of these, compounds 1, 3, and 6 were highly ranked in shape-focused virtual screening.


