AlaterninCAS# 641-90-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 641-90-7 | SDF | Download SDF |
| PubChem ID | 11818503 | Appearance | Orange powder |
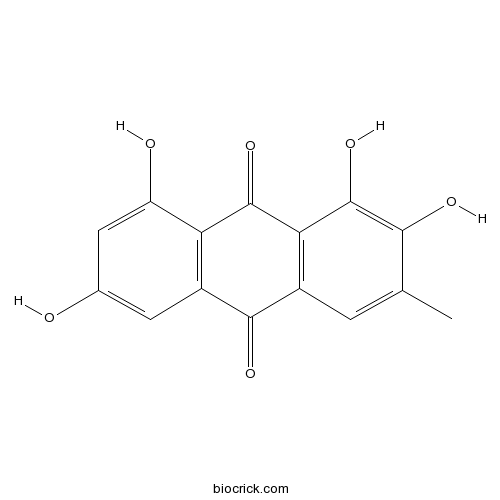
| Formula | C15H10O6 | M.Wt | 286.24 |
| Type of Compound | Anthraquinones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1,2,6,8-tetrahydroxy-3-methylanthracene-9,10-dione | ||
| SMILES | CC1=CC2=C(C(=C1O)O)C(=O)C3=C(C2=O)C=C(C=C3O)O | ||
| Standard InChIKey | LAOFTEMTSXNIIM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H10O6/c1-5-2-7-11(15(21)12(5)18)14(20)10-8(13(7)19)3-6(16)4-9(10)17/h2-4,16-18,21H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Alaternin Dilution Calculator

Alaternin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4936 mL | 17.4679 mL | 34.9357 mL | 69.8714 mL | 87.3393 mL |
| 5 mM | 0.6987 mL | 3.4936 mL | 6.9871 mL | 13.9743 mL | 17.4679 mL |
| 10 mM | 0.3494 mL | 1.7468 mL | 3.4936 mL | 6.9871 mL | 8.7339 mL |
| 50 mM | 0.0699 mL | 0.3494 mL | 0.6987 mL | 1.3974 mL | 1.7468 mL |
| 100 mM | 0.0349 mL | 0.1747 mL | 0.3494 mL | 0.6987 mL | 0.8734 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Adenostemmoside A
Catalog No.:BCN9707
CAS No.:130217-15-1
- Onjixanthone I
Catalog No.:BCN9706
CAS No.:136083-92-6
- 12-O-Methylinophyllum D
Catalog No.:BCN9705
CAS No.:40883-10-1
- Adenostemmoic acid G
Catalog No.:BCN9704
CAS No.:130217-26-4
- Indole-3-carboxylic acid β-D-glucopyranosyl ester
Catalog No.:BCN9703
CAS No.:106871-55-0
- Gancaonin J
Catalog No.:BCN9702
CAS No.:129280-37-1
- Adenostemmoic acid E
Catalog No.:BCN9701
CAS No.:130217-22-0
- 14-Benzoyl-8-O-methylaconine
Catalog No.:BCN9700
CAS No.:93772-68-0
- 2-Hydroxy-1-methoxyxanthone
Catalog No.:BCN9699
CAS No.:16302-46-8
- Anhydroscandenolide
Catalog No.:BCN9698
CAS No.:114742-71-1
- Futoamide
Catalog No.:BCN9697
CAS No.:23477-80-7
- 14-O-Acetylneoline
Catalog No.:BCN9696
CAS No.:1354-86-5
- Liquiridiolic acid
Catalog No.:BCN9709
CAS No.:20528-70-5
- Inophyllum E
Catalog No.:BCN9710
CAS No.:17312-31-1
- Isooxoflaccidin
Catalog No.:BCN9711
CAS No.:135010-50-3
- Aculeatin
Catalog No.:BCN9712
CAS No.:77636-05-6
- 4'-O-Methyllariciresinol
Catalog No.:BCN9713
CAS No.:73354-09-3
- Isodihydrocadambine
Catalog No.:BCN9714
CAS No.:55624-02-7
- 5,6,4'-Trihydroxy-3,7-dimethoxyflavone
Catalog No.:BCN9715
CAS No.:56226-95-0
- Blestriarene C
Catalog No.:BCN9716
CAS No.:120090-81-5
- Adenostemmoic acid B
Catalog No.:BCN9717
CAS No.:130217-16-2
- Hortiamide
Catalog No.:BCN9718
CAS No.:106055-13-4
- Adenostemmoic acid D
Catalog No.:BCN9719
CAS No.:130217-20-8
- Yukocitrine
Catalog No.:BCN9720
CAS No.:145940-32-5
Establishing GPCR Targets of hMAO Active Anthraquinones from Cassia obtusifolia Linn Seeds Using In Silico and In Vitro Methods.[Pubmed:32280914]
ACS Omega. 2020 Mar 25;5(13):7705-7715.
The present study examines the effect of human monoamine oxidase active anthraquinones emodin, Alaternin (=7-hydroxyemodin), aloe-emodin, and questin from Cassia obtusifolia Linn seeds in modulating human dopamine (hD1R, hD3R, and hD4R), serotonin (h5-HT1AR), and vasopressin (hV1AR) receptors that were predicted as prime targets from proteocheminformatics modeling via in vitro cell-based functional assays, and explores the possible mechanisms of action via in silico modeling. Emodin and Alaternin showed a concentration-dependent agonist effect on hD3R with EC50 values of 21.85 +/- 2.66 and 56.85 +/- 4.59 muM, respectively. On hV1AR, emodin and Alaternin showed an antagonist effect with IC50 values of 10.25 +/- 1.97 and 11.51 +/- 1.08 muM, respectively. Interestingly, questin and aloe-emodin did not have any observable effect on hV1AR. Only Alaternin was effective in antagonizing h5-HT1AR (IC50: 84.23 +/- 4.12 muM). In silico studies revealed that a hydroxyl group at C1, C3, and C8 and a methyl group at C6 of anthraquinone structure are essential for hD3R agonist and hV1AR antagonist effects, as well as for the H-bond interaction of 1-OH group with Ser192 at a proximity of 2.0 A. Thus, based on in silico and in vitro results, hV1AR, hD3R, and h5-HT1AR appear to be prime targets of the tested anthraquinones.
In Vitro and in Silico Human Monoamine Oxidase Inhibitory Potential of Anthraquinones, Naphthopyrones, and Naphthalenic Lactones from Cassia obtusifolia Linn Seeds.[Pubmed:31592482]
ACS Omega. 2019 Sep 18;4(14):16139-16152.
In recent years, Cassia seed extract has been reported as a neuroprotective agent in various models of neurodegeneration, mainly via an antioxidant mechanism. However, no one has previously reported the effects of Cassia seed extract and its phytochemicals on human monoamine oxidase (hMAO) enzyme activity. The seed methanol extract, the solvent-soluble fractions, and almost all isolated compounds displayed selective inhibition of hMAO-A isozyme activity. Interestingly, compounds obtusin (3), Alaternin (8), aloe-emodin (9), questin (12), rubrofusarin (13), cassiaside (15), toralactone 9-O-beta-gentiobioside (26), and (3S)-9,10-dihydroxy-7-methoxy-3-methyl-1-oxo-3,4-dihydro-1H-benzo[g]isochromene-3 -carboxylic acid 9-O-beta-d-glucopyranoside (38) showed the most promising inhibition of the hMAO-A isozyme with IC50 values of 0.17-11 muM. The kinetic study characterized their mode of inhibition and molecular docking simulation predicted interactions with Ile-335 and Tyr-326 in support of the substrate/inhibitor selectivity in respective isozymes. These results demonstrate that Cassia seed extract and its constituents inhibit hMAO-A enzyme activity with high selectivity and suggest that they could play a preventive role in neurodegenerative diseases, especially anxiety and depression.
Promising Inhibitory Effects of Anthraquinones, Naphthopyrone, and Naphthalene Glycosides, from Cassia obtusifolia on alpha-Glucosidase and Human Protein Tyrosine Phosphatases 1B.[Pubmed:28035984]
Molecules. 2016 Dec 27;22(1). pii: molecules22010028.
The present work aims to evaluate the anti-diabetic potentials of 16 anthraquinones, two naphthopyrone glycosides, and one naphthalene glycoside from Cassia obtusifolia via inhibition against the protein tyrosine phosphatases 1B (PTP1B) and alpha-glucosidase. Among them, anthraquinones emodin and Alaternin exhibited the highest inhibitory activities on PTP1B and alpha-glucosidase, respectively. Moreover, we examined the effects of Alaternin and emodin on stimulation of glucose uptake by insulin-resistant human HepG2 cells. The results showed that Alaternin and emodin significantly increased the insulin-provoked glucose uptake. In addition, our kinetic study revealed that Alaternin competitively inhibited PTP1B, and showed mixed-type inhibition against alpha-glucosidase. In order to confirm enzyme inhibition, we predicted the 3D structure of PTP1B using Autodock 4.2 to simulate the binding of Alaternin. The docking simulation results demonstrated that four residues of PTP1B (Gly183, Arg221, Ile219, Gly220) interact with three hydroxyl groups of Alaternin and that the binding energy was negative (-6.30 kcal/mol), indicating that the four hydrogen bonds stabilize the open form of the enzyme and potentiate tight binding of the active site of PTP1B, resulting in more effective PTP1B inhibition. The results of the present study clearly demonstrate that C. obtusifolia and its constituents have potential anti-diabetic activity and can be used as a functional food for the treatment of diabetes and associated complications.
Inhibitory activities of major anthraquinones and other constituents from Cassia obtusifolia against beta-secretase and cholinesterases.[Pubmed:27321278]
J Ethnopharmacol. 2016 Sep 15;191:152-160.
ETHNOPHARMACOLOGICAL RELEVANCE: Semen Cassiae has been traditionally used as an herbal remedy for liver, eye, and acute inflammatory diseases. Recent pharmacological reports have indicated that Cassiae semen has neuroprotective effects, attributable to its anti-inflammatory actions, in ischemic stroke and Alzheimer's disease (AD) models. AIM OF THE STUDY: The basic goal of this study was to evaluate the anti-AD activities of C. obtusifolia and its major constituents. Previously, the extract of C. obtusifolia seeds, was reported to have memory enhancing properties and anti-AD activity to ameliorate amyloid beta-induced synaptic dysfunction. However, the responsible components of C. obtusifolia seeds in an AD are currently still unknown. In this study, we investigated the inhibitory effects of C. obtusifolia and its constituents against acetylcholinesterase (AChE), butyrylcholinesterase (BChE), and beta-site amyloid precursor protein (APP) cleaving enzyme 1 (BACE1) enzyme activity. MATERIALS AND METHODS: In vitro cholinesterase enzyme assays by using AChE, BChE, and BACE1 were performed. We also scrutinized the potentials of Cassiae semen active component as BACE1 inhibitors via enzyme kinetics and molecular docking simulation. RESULTS: In vitro enzyme assays demonstrated that C. obtusifolia and its major constituents have promising inhibitory potential against AChE, BChE, and BACE1. All Cassiae semen constituents exhibited potent inhibitory activities against AChE and BACE1 with IC50 values of 6.29-109microg/mL and 0.94-190microg/mL, whereas Alaternin, questin, and toralactone gentiobioside exhibited significant inhibitory activities against BChE with IC50 values of 113.10-137.74microg/mL. Kinetic study revealed that Alaternin noncompetitively inhibited, whereas cassiaside and emodin showed mixed-type inhibition against BACE1. Furthermore, molecular docking simulation results demonstrated that hydroxyl group of Alaternin and emodin tightly interacted with the active site residues of BACE1 and their relevant binding energies (-6.62 and -6.89kcal/mol), indicating a higher affinity and tighter binding capacity of these compounds for the active site of BACE1. CONCLUSION: The findings of the present study suggest the potential of C. obtusifolia and its major constituents for use in the development of therapeutic or preventive agents for AD, especially through inhibition of AChE, BChE and BACE1 activities.
A new anthraquinone glycoside from Rhamnus nakaharai and anti-tyrosinase effect of 6-methoxysorigenin.[Pubmed:26828875]
Nat Prod Res. 2016 Dec;30(23):2655-2661.
In continual study on the heartwood of Rhamnus nakaharai, a new Alaternin-8-O-glucoside, namely 1,2,6,8-tetrahydroxy-3-methylanthraquinone-8-O-beta-glucopyranoside (1), together with some known compounds were further isolated and characterised by 1-D, 2-D NMR and other spectral evidences. The free radical scavenging and antityrosinase activities of the isolates, including Alaternin (1a), emodin (2a), emodin-8-O-beta-glucopyranoside (2), 6-methoxysorigenin-8-O-beta-glucopyranoside (3) and 6-methoxysorigenin (3a) were tested. Alaternin (1a) exhibited to be mild DPPH radical scavenger with half as potent as vitamin C, while both Alaternin (1a) and emodin-8-O-beta-glucopyranoside (2) exhibited stronger SOD-like activity than that of BHA. 6-Methoxysorigenin (3a), a reported potential antioxidant, and its 8-O-glucoside (3) both performed significant inhibitory effect on mushroom tyrosinase with about twice as potent as kojic acid, the positive control.
Alaternin attenuates delayed neuronal cell death induced by transient cerebral hypoperfusion in mice.[Pubmed:20304026]
Food Chem Toxicol. 2010 Jun;48(6):1528-36.
The aim of this study was to determine whether Alaternin exhibits neuroprotective activity after transient cerebral hypoperfusion induced by bilateral common carotid artery occlusion (BCCAO). Mice were subjected to BCCAO, and circulation was restored after 20 min. Alaternin (10 mg/kg, p.o) treatment significantly prevented nitrotyrosine and lipid peroxidation, as well as BCCAO induced-inducible nitric oxide synthase (iNOS) expression. Alaternin also significantly reduced microglial activation (a marker of inflammation). The number of viable neurons detected by Nissl staining increased with Alaternin (10 mg/kg, p.o) treatment at 7 days post-BCCAO. In the passive avoidance task, Alaternin significantly ameliorated BCCAO-induced cognitive impairments (P<0.05). These results suggest that the neuroprotective effects of Alaternin are mediated by its anti-inflammatory and radical scavenging activities.
A rubrofusarin gentiobioside isomer from roastedCassia tora.[Pubmed:18982501]
Arch Pharm Res. 1997 Oct;20(5):513-5.
From the roasted seeds ofCassia tora L., a new naphthopyrone glycoside was isolated and characterized as 10-[(beta-D-glucopyranosyl-(1-->6)-O-beta-D-glucopyranosyl)oxyl-5-hydroxy-8-metho xy-2-methyl-4H-naphtho [1,2-b]pyran-4-one(isorubrofusarin gentiobioside). Along with isorubrofusarin gentiobioside, Alaternin and adenosine were isolated and identified.
Inhibitory activities of Cassia tora and its anthraquinone constituents on angiotensin-converting enzyme.[Pubmed:18803227]
Phytother Res. 2009 Feb;23(2):178-84.
As a component of our program that pertains to the isolation of antihypertensive agents derived from natural products, we screened the bioactivity of seeds from raw and roasted Cassia tora via angiotensin converting enzyme (ACE) inhibitory assays. We found that both of the MeOH extracts from the raw and roasted C. tora exhibited significant inhibitory properties against ACE, demonstrating more than 50% inhibition at a concentration of 163.93 microg/mL. Emodin (3), Alaternin (4), gluco-obtusifolin (5), cassiaside (6), gluco-aurantioobtusin (7), cassitoroside (8), toralactone gentiobioside (9), and chrysophanol triglucoside (10) had been previously isolated; however, questin (1) and 2-hydroxyemodin 1-methylether (2) were isolated from C. tora for the first time in this study. Among them, only anthraquinone glycoside (7) demonstrated marked inhibitory activity against ACE, with an IC(50) value of 30.24 +/- 0.20 microM. Conversely, aurantioobtusin (7a), obtained from the acid hydrolysis of 7, showed no activity. Further inhibitory kinetics analyzed from Lineweaver-Burk plots showed 7 to be a competitive inhibitor with a Ki value of 8.3 x 10(-5) M. Moreover, compound 7 showed marked inhibitory and scavenging activities with an IC(50) value of 49.64 +/- 0.37 microM (positive control; trolox: 26.07 +/- 1.05 microM) for total reactive oxygen species generation, and 4.60 +/- 1.12 microM (positive control; penicillamine: 0.24 +/- 0.04 microM) for ONOO(-).
Peroxynitrite scavenging mode of alaternin isolated from Cassia tora.[Pubmed:15482647]
J Pharm Pharmacol. 2004 Oct;56(10):1315-21.
Peroxynitrite (ONOO-), formed from the reaction of superoxide (.O2-) and nitric oxide (NO), is a potent oxidant that contributes to the oxidation of various cellular constituents, including lipids, amino acids, sulfhydryls and nucleotides. It can cause cellular injury, such as DNA fragmentation and apoptotic cell death. ONOO- toxicity is also reported to be involved in inflammatory and neurodegenerative diseases, such as Alzheimer's disease, Parkinson's disease and atherosclerosis. Moreover, the necessity for a strong ONOO- scavenger is important because of the lack of endogenous enzymes that protect against the damage caused by ONOO-. The aim of this study was to evaluate the ability of natural products to scavenge ONOO-. We tested various plant extracts for their ONOO- scavenging activity. Among them, extract from Cassia tora, which is well known as an oriental herb in traditional medicine, showed potent ONOO- scavenging activity. Further analysis identified the phenolic active components, Alaternin and nor-rubrofusarin glucose, as potent ONOO- scavengers. Spectrophotometric analysis demonstrated that Alaternin and nor-rubrofusarin glucose led to a decrease in the ONOO- -mediated nitration of tyrosine through electron donation. In bovine serum albumin, Alaternin, but not nor-rubrofusarin glucose, showed significant inhibition of ONOO- -mediated nitration in a dose-dependent manner. We believe Alaternin can be developed as an effective ONOO- scavenger for the prevention of ONOO- -associated diseases.
Alaternin and emodin with hydroxyl radical inhibitory and/or scavenging activities and hepatoprotective activity on tacrine-induced cytotoxicity in HepG2 cells.[Pubmed:15473666]
Arch Pharm Res. 2004 Sep;27(9):947-53.
The antioxidative and hepatoprotective potentials of two anthraquinones, Alaternin (2-hydroxyemodin) and emodin, to scavenge and/or inhibit hydroxyl radicals generated by the Fenton reaction and to protect tacrine-induced cytotoxicity in human liver derived HepG2 cells were evaluated, respectively. The inhibitory activity on hydroxyl radical generated in a cell-free chemical system (FeSO4/H2O2) was investigated by a fluorescence spectrophotometer using a highly fluorescent probe, 2',7'-dichlorofluorescein. The hydroxyl radical scavenging activity was determined by electron spin resonance spectroscopy using 5,5-dimethy-1-pyrroline-N-oxide as hydroxyl radicals trapping agents. Tacrine-induced HepG2 cell toxicity was determined by a 3-[4,5-dimethylthiazole-2yl]-2,5-diphenyltertrazolium bromide assay. Although the scavenging activity of Alaternin on hydroxyl radical was similar to that of emodin in dose-dependent patterns, the inhibitory activity exhibited by the former on hydroxyl radical generation was stronger than that of the latter, with IC50 values of 3.05 +/- 0.26 microM and 13.29 +/- 3.20 microM, respectively. In addition, the two anthraquinones, Alaternin and emodin showed their hepatoprotective activities on tacrine-induced cytotoxicity, and the EC50 values were 4.02 microM and 2.37 microM, respectively. Silymarin, an antihepatotoxic agent used as a positive control exhibited the EC50 value of 2.00 microM. These results demonstrated that both Alaternin and emodin had the simultaneous antioxidant and hepatoprotective activities.


