AculeatinCAS# 77636-05-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 77636-05-6 | SDF | Download SDF |
| PubChem ID | 101628361 | Appearance | Powder |
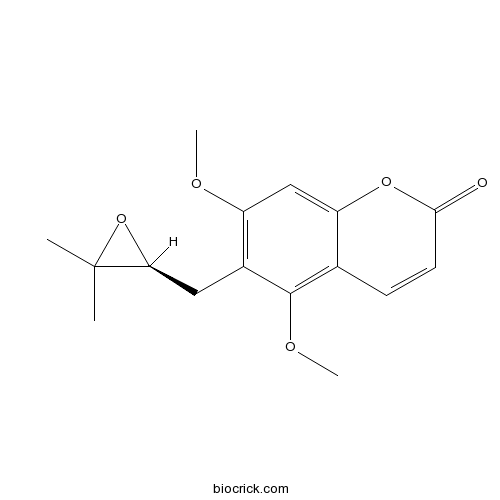
| Formula | C16H18O5 | M.Wt | 290.31 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 6-[[(2S)-3,3-dimethyloxiran-2-yl]methyl]-5,7-dimethoxychromen-2-one | ||
| SMILES | CC1(C(O1)CC2=C(C=C3C(=C2OC)C=CC(=O)O3)OC)C | ||
| Standard InChIKey | DZSSBQWTSOMKDI-ZDUSSCGKSA-N | ||
| Standard InChI | InChI=1S/C16H18O5/c1-16(2)13(21-16)7-10-11(18-3)8-12-9(15(10)19-4)5-6-14(17)20-12/h5-6,8,13H,7H2,1-4H3/t13-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Aculeatin Dilution Calculator

Aculeatin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4446 mL | 17.223 mL | 34.4459 mL | 68.8919 mL | 86.1148 mL |
| 5 mM | 0.6889 mL | 3.4446 mL | 6.8892 mL | 13.7784 mL | 17.223 mL |
| 10 mM | 0.3445 mL | 1.7223 mL | 3.4446 mL | 6.8892 mL | 8.6115 mL |
| 50 mM | 0.0689 mL | 0.3445 mL | 0.6889 mL | 1.3778 mL | 1.7223 mL |
| 100 mM | 0.0344 mL | 0.1722 mL | 0.3445 mL | 0.6889 mL | 0.8611 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isooxoflaccidin
Catalog No.:BCN9711
CAS No.:135010-50-3
- Inophyllum E
Catalog No.:BCN9710
CAS No.:17312-31-1
- Liquiridiolic acid
Catalog No.:BCN9709
CAS No.:20528-70-5
- Alaternin
Catalog No.:BCN9708
CAS No.:641-90-7
- Adenostemmoside A
Catalog No.:BCN9707
CAS No.:130217-15-1
- Onjixanthone I
Catalog No.:BCN9706
CAS No.:136083-92-6
- 12-O-Methylinophyllum D
Catalog No.:BCN9705
CAS No.:40883-10-1
- Adenostemmoic acid G
Catalog No.:BCN9704
CAS No.:130217-26-4
- Indole-3-carboxylic acid β-D-glucopyranosyl ester
Catalog No.:BCN9703
CAS No.:106871-55-0
- Gancaonin J
Catalog No.:BCN9702
CAS No.:129280-37-1
- Adenostemmoic acid E
Catalog No.:BCN9701
CAS No.:130217-22-0
- 14-Benzoyl-8-O-methylaconine
Catalog No.:BCN9700
CAS No.:93772-68-0
- 4'-O-Methyllariciresinol
Catalog No.:BCN9713
CAS No.:73354-09-3
- Isodihydrocadambine
Catalog No.:BCN9714
CAS No.:55624-02-7
- 5,6,4'-Trihydroxy-3,7-dimethoxyflavone
Catalog No.:BCN9715
CAS No.:56226-95-0
- Blestriarene C
Catalog No.:BCN9716
CAS No.:120090-81-5
- Adenostemmoic acid B
Catalog No.:BCN9717
CAS No.:130217-16-2
- Hortiamide
Catalog No.:BCN9718
CAS No.:106055-13-4
- Adenostemmoic acid D
Catalog No.:BCN9719
CAS No.:130217-20-8
- Yukocitrine
Catalog No.:BCN9720
CAS No.:145940-32-5
- N-Methoxy-3-hydroxymethylcarbazole
Catalog No.:BCN9721
CAS No.:142768-49-8
- N-Methoxy-3-formylcarbazole
Catalog No.:BCN9722
CAS No.:117592-01-5
- O-Methylmurrayamine A
Catalog No.:BCN9723
CAS No.:134779-20-7
- Stephodeline
Catalog No.:BCN9724
CAS No.:56596-12-4
Evaluation of Aculeatin and Toddaculin Isolated from Toddalia asiatica as Anti-inflammatory Agents in LPS-Stimulated RAW264 Macrophages.[Pubmed:29311475]
Biol Pharm Bull. 2018;41(1):132-137.
Anti-inflammatory activity of Aculeatin and toddaculin, which are coumarins with a similar structure isolated from Toddalia asiatica (L.) LAM., was evaluated using lipopolysaccharide (LPS)-stimulated RAW264 mouse macrophage cells. Both Aculeatin and toddaculin significantly inhibited mRNA expression of inflammatory mediators and nitric oxide production. Furthermore, Toddaculin suppressed LPS-induced phosphorylation of p38 and extracellular signal-regulated kinase (ERK)1/2 and inhibited LPS-induced activation of nuclear factor-kappaB (NF-kappaB). However, Aculeatin did not exhibit such effects, suggesting that Aculeatin and toddaculin suppress LPS-induced inflammation of RAW264 cells via different mechanisms. The cellular uptake of these compounds was also evaluated. Toddaculin was detected in RAW264 cells after 4 and 24 h. However, Aculeatin levels were not observed in RAW264 cells at all incubation intervals. These results indicate that de-epoxidation of a prenyl group can increase hydrophobicity of molecule and is thought to accelerate cellular uptake and/or interactions with the phospholipid bilayers of cell membranes.
Uncovering new structural insights for antimalarial activity from cost-effective aculeatin-like derivatives.[Pubmed:25519040]
Org Biomol Chem. 2015 Feb 21;13(7):2064-77.
A series of new Aculeatin-like analogues were synthesized in two steps by combining two sets of building blocks. Many compounds showed inhibitory activities in vitro against Plasmodium falciparum and have helped to gain more insight into structure-activity relationships around the spirocyclohexadienone pharmacophoric scaffold. Plasmodium falciparum thioredoxin reductase (PfTrxR) has been investigated as a putative cellular target. Moreover, a new Aculeatin-like scaffold without Michael acceptor properties, efficient at 0.86 muM against P. falciparum 3D7, was identified and raises the prospect of developing a new antimalarial agent.
Aculeatin, a coumarin derived from Toddalia asiatica (L.) Lam., enhances differentiation and lipolysis of 3T3-L1 adipocytes.[Pubmed:25445590]
Biochem Biophys Res Commun. 2014 Oct 31;453(4):787-92.
Toddalia asiatica (L.) Lam. (T. asiatica) has been utilized traditionally for medicinal purposes such as the treatment of diabetes. Currently, the extract is considered to be a good source of anti-diabetic agents, but the active compounds have yet to be identified. In this study, we investigated the effects of fractionated T. asiatica extracts on the differentiation of 3T3-L1 preadipocytes and identified Aculeatin as a potential active agent. When 3T3-L1 preadipocytes were treated with Aculeatin isolated from T. asiatica in the presence of insulin, Aculeatin increased cellular triglyceride levels and glycerol-3-phosphate dehydrogenase activity. This indicated that Aculeatin could enhance the differentiation of preadipocytes into adipocytes. Further analyses using a DNA microarray and real-time quantitative reverse-transcription PCR showed an increase in the expression of peroxisome proliferator-activated receptor-gamma target genes (Pparg, Ap2, Cd36, Glut4 and Adipoq) by Aculeatin, suggesting that Aculeatin enhances the differentiation of 3T3-L1 cells by modulating the expression of genes critical for adipogenesis. Interestingly, after treatment of differentiated adipocytes with Aculeatin, glucose uptake and lipolysis were enhanced. Overall, our results suggested that Aculeatin is an active compound in T. asiatica for enhancing both differentiation and lipolysis of adipocytes, which are useful for the treatment of lipid abnormalities as well as diabetes.
Total synthesis of aculeatin A via double intramolecular oxa-Michael addition of secondary/tertiary alcohols.[Pubmed:24393066]
J Org Chem. 2014 Feb 7;79(3):1498-504.
A new synthetic strategy was developed for a concise total synthesis of Aculeatin A as a single spiroisomer in both racemic and enantioselective fashions in 8-10 steps with approximately 10% overall yield from the known alkyne 11, featuring phenol oxidative dearomatization, double intramolecular oxa-Michael addition of secondary/tertiary alcohols, and chemo- and stereoselective reduction of ketone. The new synthetic strategy greatly expedites the access to the potent antiprotozoal Aculeatin A, 6-epi-Aculeatin D, and their analogues.
Stereoselective synthesis of bioactive natural spiroacetals aculeatins A and B.[Pubmed:20194022]
Bioorg Med Chem Lett. 2010 Apr 1;20(7):2303-5.
The stereoselective synthesis of two naturally occurring bioactive spiroacetals, Aculeatins A and B has been accomplished using 1-tetradecanal as the starting material. The sequence introduces diastereoselective iodine-induced electrophilic cyclization and ring opening of epoxide with 1,3-dithiane as the key steps.
Polyol synthesis with beta-oxyanionic alkyllithium reagents: syntheses of aculeatins A, B, and D.[Pubmed:19691310]
Org Lett. 2009 Sep 17;11(18):4220-3.
Synthesis of ketone aldol products using a non-aldol route was developed. The beta-phenylthio alcohols were prepared from optically pure oxiranes. Deprotonation and reductive lithiation generated the key intermediate, a beta-oxyanionic alkyllithium reagent. Addition to a Weinreb amide produced the beta-hydroxy ketone in >90% yield using only 1.5 equiv of the phenylthio alcohol. Stereoselective reduction of the ketone led to either the syn- or anti-1,3-diol. This simple, convergent sequence was used to prepare Aculeatins A, B, and D from a common intermediate.
A chiron approach to the total synthesis of (+)-aculeatin D.[Pubmed:18707170]
J Org Chem. 2008 Sep 19;73(18):7310-6.
A synthesis of natural Aculeatin D has been achieved, with the key stereogenic centers taken from inexpensive and readily available D-xylose. In elaboration of D-xylose into a desired form readily applicable in synthesis a previously misinterpreted and overlooked abnormal selectivity in hydroxyl protection was noticed and exploited. Protocols were developed for monotosylation of a triol insoluble in CH2Cl2 and "freezing" the less stable isomer (Aculeatin D) at the PIFA-mediated oxidative spirocyclization, respectively. An unexplained deprotonation at a benzyl protecting group by a thermodynamically more stable dithiane carbanion in the literature was also addressed.
Enhanced antimalarial activity of novel synthetic aculeatin derivatives.[Pubmed:18680278]
J Med Chem. 2008 Aug 28;51(16):4870-3.
We report the design, synthesis, and in vitro evaluation of novel polyspirocyclic structures, inspired by the antimalarial natural products, the Aculeatins. A divergent synthetic strategy was conceived for the practical supply and has allowed the discovery of two novel and more potent analogues active on the Plasmodium falciparum 3D7 strain. Moreover, these compounds proved to be potent against Toxoplasma gondii. A number of features that govern these inhibitions were identified.
A carbohydrate-based approach for the total synthesis of aculeatin D and 6-epi-aculeatin D.[Pubmed:18412320]
J Org Chem. 2008 May 16;73(10):3915-8.
A concise approach for the total synthesis of Aculeatin D and 6-epi-Aculeatin D employing differentially protected anti, anti-1,3,5-triol alkyne prepared from alpha-D-glucoheptonic-gamma-lactone derivative is documented. Phenol protecting group manipulation for selective O-debenzylation during the hydrogenation of the diyne intermediate and one-pot phenolic oxidation with concomitant spiroketalization highlight the accomplished total synthesis.
Potential anticancer activity of naturally occurring and semisynthetic derivatives of aculeatins A and B from Amomum aculeatum.[Pubmed:18260638]
J Nat Prod. 2008 Mar;71(3):390-5.
Activity-guided fractionation of hexanes- and CHCl 3-soluble extracts of Amomum aculeatum leaves, collected in Indonesia, led to the isolation of three new dioxadispiroketal-type ( 3- 5) and two new oxaspiroketal-type ( 6 and 7) derivatives. Nine semisynthetic derivatives ( 1a- 1h and 2a) of the parent compounds, Aculeatins A ( 1) and B ( 2), were prepared. All isolates and semisynthetic compounds were tested against a small panel of human cell lines. Of these, Aculeatin A ( 1; ED 50 0.2-1.0 microM) was found to be among the most cytotoxic of the compounds tested and was further evaluated in an in vivo hollow fiber assay; it was found to be active against MCF-7 (human breast cancer) cells implanted intraperitoneally at doses of 6.25, 12.5, 25, and 50 mg/kg. However, when 1 was tested using P388 lymphocytic leukemia and human A2780 ovarian carcinoma in vivo models, it was deemed to be inactive at the doses used.
Synthesis of (+/-)-aculeatins A and B.[Pubmed:12119675]
Chem Commun (Camb). 2002 Apr 7;(7):686-7.
The first synthesis of two new antiprotozoal and natural products was performed using concomitant deprotecting dithiane-phenolic oxidative reactions to form in one-step the 1,7-dioxadispiro[5.1.5.2]pentadecane core.
Minor cytotoxic and antibacterial compounds from the rhizomes of Amomum aculeatum.[Pubmed:11454360]
Phytochemistry. 2001 Aug;57(8):1281-5.
A new cytotoxic 1,7-dioxa-dispiro[5.1.5.2]pentadeca-9,12-dien-11-one derivative, Aculeatin D, and a new alkenone, 5-hydroxy-hexacos-1-en-3-one, have been isolated as minor compounds from the rhizomes of Amomum aculeatum. Their structures have been determined mainly by NMR spectroscopy and mass spectrometry. Aculeatin D showed high cytotoxicity against the KB and the L-6 cell line with IC(50) of 0.38 microg/ml and 1 microg/ml, respectively. Additionally, it revealed remarkable activity against two Plasmodium falciparum strains, as well as against Trypanosoma brucei rhodesiense and Trypanosoma cruzi. 5-Hydroxy-hexacos-1-en-3-one exhibited neither cytotoxic nor antiprotozoal activity, whereas antibacterial testing against Bacillus cereus, Escherichia coli and Staphylococcus epidermidis showed moderate to strong activity for both compounds.
[Studies on the chemical constituents of rutaceous plants. LXVII. The chemical constituents of Toddalia asiatica (L.) Lam. (T. aculeata Pers). Examination of coumarins using supercritical fluid and soxhlet extraction. Is toddalolactone a genuine natural coumarin?].[Pubmed:1783986]
Yakugaku Zasshi. 1991 Jul;111(7):376-85.
It is well known that toddalolactone (1) is a main component of Toddalia asiatica (L.) Lam. (T. aculeata Pers.) (Rutaceae). However, supercritical fluid (SCF) extraction of the plant by using CO2 showed that a main component of the extract was not 1, but Aculeatin (2), a coumarin having an epoxy ring on the side chain. The same result was obtained from Soxhlet extraction by using aprotic solvents. On the other hand, Soxhlet extraction by using methanol yielded 13, corresponding to a methanol adduct of 2, as an additional component, which was able to be also produced in 50.2% yield only by heating pure 2 in methanol, indicating that the epoxy ring in 2 can be easily attacked by a weak nucleophile like methanol. These facts strongly suggested that 1, corresponding to the hydrate of 2, was an artefact derived from 2 during extraction. SCF extraction under various conditions was examined in detail by quantitative analyses of 1 and 2 by high performance liquid chromatography and the optimum condition extracting the both components was found to be at 40 degrees C and at 300 kg/cm2. The condition was applied to the plant treated with aqueous sodium hydrogen carbonate in order to remove any acidic substances and 1 was still detected in the extract. Thus, it is conclude that 1 should be a genuine natural coumarin but that previous isolation of 1 as a main component resulted in an isolation of an artefact derived from 2. SCF extraction was suggested to be a useful extraction method.


