O-Methylmurrayamine ACAS# 134779-20-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 134779-20-7 | SDF | Download SDF |
| PubChem ID | 14892681 | Appearance | Powder |
| Formula | C19H19NO2 | M.Wt | 293.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
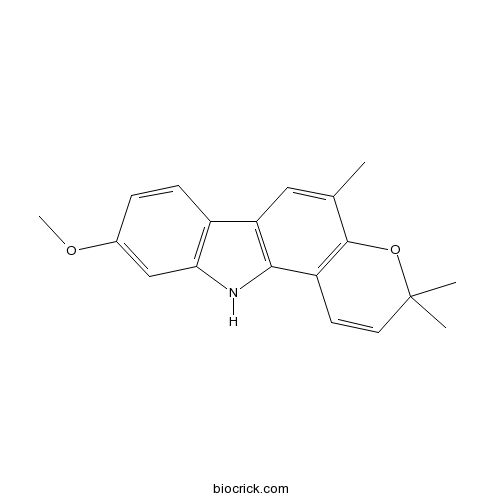
| Chemical Name | 9-methoxy-3,3,5-trimethyl-11H-pyrano[3,2-a]carbazole | ||
| SMILES | CC1=CC2=C(C3=C1OC(C=C3)(C)C)NC4=C2C=CC(=C4)OC | ||
| Standard InChIKey | GDQWCFKJPYSDDG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H19NO2/c1-11-9-15-13-6-5-12(21-4)10-16(13)20-17(15)14-7-8-19(2,3)22-18(11)14/h5-10,20H,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

O-Methylmurrayamine A Dilution Calculator

O-Methylmurrayamine A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4083 mL | 17.0416 mL | 34.0832 mL | 68.1663 mL | 85.2079 mL |
| 5 mM | 0.6817 mL | 3.4083 mL | 6.8166 mL | 13.6333 mL | 17.0416 mL |
| 10 mM | 0.3408 mL | 1.7042 mL | 3.4083 mL | 6.8166 mL | 8.5208 mL |
| 50 mM | 0.0682 mL | 0.3408 mL | 0.6817 mL | 1.3633 mL | 1.7042 mL |
| 100 mM | 0.0341 mL | 0.1704 mL | 0.3408 mL | 0.6817 mL | 0.8521 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- N-Methoxy-3-formylcarbazole
Catalog No.:BCN9722
CAS No.:117592-01-5
- N-Methoxy-3-hydroxymethylcarbazole
Catalog No.:BCN9721
CAS No.:142768-49-8
- Yukocitrine
Catalog No.:BCN9720
CAS No.:145940-32-5
- Adenostemmoic acid D
Catalog No.:BCN9719
CAS No.:130217-20-8
- Hortiamide
Catalog No.:BCN9718
CAS No.:106055-13-4
- Adenostemmoic acid B
Catalog No.:BCN9717
CAS No.:130217-16-2
- Blestriarene C
Catalog No.:BCN9716
CAS No.:120090-81-5
- 5,6,4'-Trihydroxy-3,7-dimethoxyflavone
Catalog No.:BCN9715
CAS No.:56226-95-0
- Isodihydrocadambine
Catalog No.:BCN9714
CAS No.:55624-02-7
- 4'-O-Methyllariciresinol
Catalog No.:BCN9713
CAS No.:73354-09-3
- Aculeatin
Catalog No.:BCN9712
CAS No.:77636-05-6
- Isooxoflaccidin
Catalog No.:BCN9711
CAS No.:135010-50-3
- Stephodeline
Catalog No.:BCN9724
CAS No.:56596-12-4
- Rossicaside B
Catalog No.:BCN9725
CAS No.:80458-55-5
- Pumiloside
Catalog No.:BCN9726
CAS No.:126722-26-7
- Phyllanthurinolactone
Catalog No.:BCN9727
CAS No.:168180-12-9
- Artanomaloide
Catalog No.:BCN9728
CAS No.:112823-41-3
- 4-(2,6,6-Trimethyl-1-cyclohexenyl)-3-buten-2-one
Catalog No.:BCN9909
CAS No.:79-77-6
- 11-Oxomogroside IIIE
Catalog No.:BCN9730
CAS No.:2096516-68-4
- Rauhimbine
Catalog No.:BCN9731
CAS No.:66634-44-4
- Carvacryl acetate
Catalog No.:BCN9732
CAS No.:6380-28-5
- Furfuryl alcohol
Catalog No.:BCN9733
CAS No.:98-00-0
- gamma-Nonanolactone
Catalog No.:BCN9734
CAS No.:104-61-0
- 1-(3',5'-dimethoxy)phenyl-2-[4''-O-beta-D-glucopyranosyl (6->1)-O-alpha-L-rhamnopyranosyl]phenylethane
Catalog No.:BCN9735
CAS No.:1338076-61-1
Anti-colon cancer activity of Murraya koenigii leaves is due to constituent murrayazoline and O-methylmurrayamine A induced mTOR/AKT downregulation and mitochondrial apoptosis.[Pubmed:28675857]
Biomed Pharmacother. 2017 Sep;93:510-521.
In recent years, many alkaloids of plant origin have attracted great attention due to their diverse range of biological properties including anti-hyperglycemic, anti-oxidant, anti-inflammatory, anti-diabetic and anti-tumor activity. Herein, the pyranocarbazole alkaloids were isolated from leaves of Murraya koenigii and their anti-cancer potential was investigated in different cancer cell lines. Among all tested compounds, murrayazoline and O-Methylmurrayamine A demonstrated potent anti-cancer activity against DLD-1 colon cancer cells with the IC50 values of 5.7muM and 17.9muM, respectively, without any non-specific cytotoxicity against non-cancer HEK-293 and HaCaT cells. Further, studies of pure compounds revealed that the anti-cancer activity of compounds corresponds with altered cellular morphology, cell cycle arrest in G2/M phase, reactive oxygen species level and mitochondrial membrane depolarization of colon cancer cells. In addition, these compounds activated caspase-3 protein and upregulated Bax/Bcl-2 protein expression ratio leading to induction of caspase-dependent apoptosis in DLD-1 cells. These event induced by carbazole alkaloids also coincides with downregulation of Akt/mTOR suggesting downstream targeting of cell survival pathway. Thus, our in vitro studies not only provided scientific basis of the use of M. koenigii leaves in the traditional Indian Ayurveda medicines, but also expands possibilities of medicinal uses of M. koenigii leaves against colon cancer. Particularly, these findings will help in further investigating murrayazoline and O-Methylmurrayamine A or their improvised derivatives as new therapeutics for the treatment of colon cancer.
Total synthesis and neuroprotective effect of O-methylmurrayamine A and 7-methoxymurrayacine.[Pubmed:28508668]
J Asian Nat Prod Res. 2017 Jun;19(6):623-629.
O-Methylmurrayamine A (7) and 7-methoxymurrayacine (8) are natural products isolated from Murraya koenigii and Murraya siamensis, respectively. In this paper, we report the synthesis of 7 and 8 which are featured in the key step of cyclization to form carbazole intermediate 5 with mild conditions. The structures were confirmed by (1)H NMR, (13)C NMR, and HR-ESI-MS. In addition, compounds 7 and 8 were tested for their neuroprotective effects against H2O2-induced PC12 cell damage. The results showed that compounds 7 and 8 have neuroprotective effect.
Four new carbazole alkaloids from Murraya koenigii that display anti-inflammatory and anti-microbial activities.[Pubmed:26947457]
Org Biomol Chem. 2016 Mar 28;14(12):3322-32.
In our present study, four new, designated as murrayakonine A-D (), along with 18 known carbazole alkaloids were isolated from CHCl3 : MeOH (1 : 1) crude extracts of the stems and leaves of Murraya koenigii (Linn.) Spreng. The structures of the all isolated compounds were characterized by analysis of HR-ESI-MS and NMR (1D and 2D spectroscopy) results, and comparison of their data with the literature data. For the first time, all the isolates were evaluated for their anti-inflammatory activities, using both in vitro and in vivo experiments, against the key inflammatory mediators TNF-alpha and IL-6. The new compound murrayakonine A (), O-Methylmurrayamine A () and murrayanine () were proven to be the most active, efficiently inhibiting TNF-alpha and IL-6 release in a dose-dependent manner and showing decreased LPS induced TNF-alpha and IL-6 production in human PBMCs [corrected]. Furthermore, all the isolates were screened for their antimicrobial potential, and the compounds girinimbine () (IC50 3.4 muM) and 1-hydroxy-7-methoxy-8-(3-methylbut-2-en-1-yl)-9H-carbazole-3-carbaldehyde () (IC50 10.9 muM) displayed potent inhibitory effects against Bacillus cereus. Furthermore, compounds murrayamine J () (IC50 11.7 muM) and koenimbine () (IC50 17.0 muM) were active against Staphylococcus aureus. However, none of the compounds were found to be active against Escherichia coli or Candida albicans.
Total syntheses of murrayamine E, I, and K.[Pubmed:25915067]
J Org Chem. 2015 Jun 5;80(11):5666-73.
We describe efficient synthetic routes to murrayamine A (mukoenine C), O-Methylmurrayamine A, mahanine, O-methylmahanine, and murrayamine D and the first total syntheses of murrayamine E, I, and K. Key steps are a palladium-catalyzed construction of the carbazole framework and an annulation of the pyran ring, which is either catalyzed by phenylboronic acid or promoted by a Lewis acid.
Palladium(II)-catalysed total synthesis of naturally occurring pyrano[3,2-a]carbazole and pyrano[2,3-b]carbazole alkaloids.[Pubmed:24788002]
Org Biomol Chem. 2014 Jun 21;12(23):3866-76.
Seven naturally occurring pyranocarbazole alkaloids (pyrayafoline A-E, O-Methylmurrayamine A and O-methylmahanine) have been obtained by total synthesis using a palladium(II)-catalysed oxidative cyclisation of a diarylamine to an orthogonally diprotected 2,7-dihydroxycarbazole as key step.
Efficient iron-mediated approach to pyrano[3,2-a]carbazole alkaloids--first total syntheses of O-methylmurrayamine A and 7-methoxymurrayacine, first asymmetric synthesis and assignment of the absolute configuration of (-)-trans-dihydroxygirinimbine.[Pubmed:21279228]
Org Biomol Chem. 2011 Apr 7;9(7):2057-61.
Iron-mediated oxidative cyclisation provides an efficient approach to pyrano[3,2-a]carbazole alkaloids. Thus, improved routes to girinimbine and murrayacine as well as the first total syntheses of O-Methylmurrayamine A and 7-methoxymurrayacine are reported. Asymmetric epoxidation of girinimbine led to (-)-trans-dihydroxygirinimbine and the assignment of its absolute configuration.
Comparison of antioxidative properties of carbazole alkaloids from Murraya koenigii leaves.[Pubmed:14558763]
J Agric Food Chem. 2003 Oct 22;51(22):6461-7.
A new dimeric carbazole alkaloid, 8,10'-[3,3',11,11'-tetrahydro-9,9'-dihydroxy-3,3',5,8'-tetramethyl-3,3'-bis(4-met hyl-3-pentenyl)]bipyrano[3,2-a]carbazole (12), was isolated from the CH(2)Cl(2) extract of Murraya koenigii together with six known carbazole alkaloids, koenimbine (6), O-Methylmurrayamine A (7), O-methylmahanine (8), isomahanine (9), bismahanine (10), and bispyrayafoline (11). Their structures were determined on the basis of (1)H and (13)C NMR spectroscopic and mass spectrometric (MS) data. The antioxidative properties of 12 carbazole alkaloids isolated from leaves of M. koenigii were evaluated on the basis of the oil stability index together with their radical scavenging ability against 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical. On the basis of the lag time to reach a steady state, the 12 carbazoles were classified into three groups. It is suggested that an aryl hydroxyl substituent on the carbazole rings plays a role in stabilizing the thermal oxidation and rate of reaction against DPPH radical.


