Blestriarene CCAS# 120090-81-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 120090-81-5 | SDF | Download SDF |
| PubChem ID | 9982511 | Appearance | Powder |
| Formula | C30H22O6 | M.Wt | 478.5 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
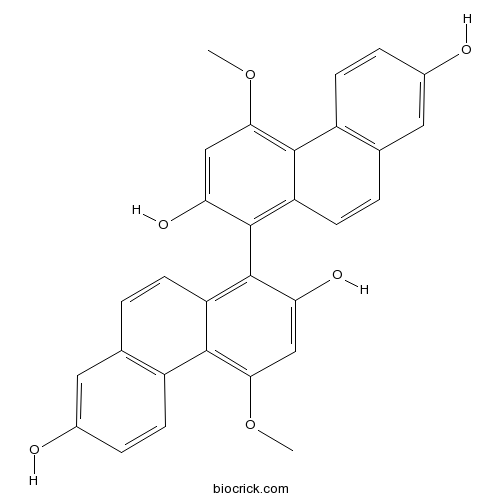
| Chemical Name | 1-(2,7-dihydroxy-4-methoxyphenanthren-1-yl)-4-methoxyphenanthrene-2,7-diol | ||
| SMILES | COC1=C2C3=C(C=CC2=C(C(=C1)O)C4=C5C=CC6=C(C5=C(C=C4O)OC)C=CC(=C6)O)C=C(C=C3)O | ||
| Standard InChIKey | CNLFNDMTGHNGIV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C30H22O6/c1-35-25-13-23(33)29(21-7-3-15-11-17(31)5-9-19(15)27(21)25)30-22-8-4-16-12-18(32)6-10-20(16)28(22)26(36-2)14-24(30)34/h3-14,31-34H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Blestriarene C Dilution Calculator

Blestriarene C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0899 mL | 10.4493 mL | 20.8986 mL | 41.7973 mL | 52.2466 mL |
| 5 mM | 0.418 mL | 2.0899 mL | 4.1797 mL | 8.3595 mL | 10.4493 mL |
| 10 mM | 0.209 mL | 1.0449 mL | 2.0899 mL | 4.1797 mL | 5.2247 mL |
| 50 mM | 0.0418 mL | 0.209 mL | 0.418 mL | 0.8359 mL | 1.0449 mL |
| 100 mM | 0.0209 mL | 0.1045 mL | 0.209 mL | 0.418 mL | 0.5225 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5,6,4'-Trihydroxy-3,7-dimethoxyflavone
Catalog No.:BCN9715
CAS No.:56226-95-0
- Isodihydrocadambine
Catalog No.:BCN9714
CAS No.:55624-02-7
- 4'-O-Methyllariciresinol
Catalog No.:BCN9713
CAS No.:73354-09-3
- Aculeatin
Catalog No.:BCN9712
CAS No.:77636-05-6
- Isooxoflaccidin
Catalog No.:BCN9711
CAS No.:135010-50-3
- Inophyllum E
Catalog No.:BCN9710
CAS No.:17312-31-1
- Liquiridiolic acid
Catalog No.:BCN9709
CAS No.:20528-70-5
- Alaternin
Catalog No.:BCN9708
CAS No.:641-90-7
- Adenostemmoside A
Catalog No.:BCN9707
CAS No.:130217-15-1
- Onjixanthone I
Catalog No.:BCN9706
CAS No.:136083-92-6
- 12-O-Methylinophyllum D
Catalog No.:BCN9705
CAS No.:40883-10-1
- Adenostemmoic acid G
Catalog No.:BCN9704
CAS No.:130217-26-4
- Adenostemmoic acid B
Catalog No.:BCN9717
CAS No.:130217-16-2
- Hortiamide
Catalog No.:BCN9718
CAS No.:106055-13-4
- Adenostemmoic acid D
Catalog No.:BCN9719
CAS No.:130217-20-8
- Yukocitrine
Catalog No.:BCN9720
CAS No.:145940-32-5
- N-Methoxy-3-hydroxymethylcarbazole
Catalog No.:BCN9721
CAS No.:142768-49-8
- N-Methoxy-3-formylcarbazole
Catalog No.:BCN9722
CAS No.:117592-01-5
- O-Methylmurrayamine A
Catalog No.:BCN9723
CAS No.:134779-20-7
- Stephodeline
Catalog No.:BCN9724
CAS No.:56596-12-4
- Rossicaside B
Catalog No.:BCN9725
CAS No.:80458-55-5
- Pumiloside
Catalog No.:BCN9726
CAS No.:126722-26-7
- Phyllanthurinolactone
Catalog No.:BCN9727
CAS No.:168180-12-9
- Artanomaloide
Catalog No.:BCN9728
CAS No.:112823-41-3
Photoracemization of Blestriarene C and Its Analogs.[Pubmed:25944278]
Chirality. 2015 Aug;27(8):479-86.
Two analogs of Blestriarene C (4,4'-dimethoxy-1,1'-biphenanthrene-2,2',7,7'-tetraol) bearing no 7,7'-dihydroxy (3) and 4,4'-dimethoxy groups were prepared. Unlike Blestriarene C (1), compounds and , as well as 1,1'-biphenanthrene-2,2'-diol (5), do not racemize under fluorescent lamp illumination. Cyclic voltammetry analysis reveals that compound has a lower half-wave potential (E(1/2)) than compounds , suggesting that a redox cycle is involved in the racemization. Compound racemizes by absorbing UV light corresponding to the (1) L(b) band. During the reaction, no side products are observed. The racemization is significantly inhibited under nitrogen. Based on these observations, we propose a feasible mechanism for the easy racemization of compound , which is mediated by a cation radical generated in situ by a reversible photo-induced oxygen oxidation.
Synthesis, resolution, and absolute stereochemistry of (-)-blestriarene C.[Pubmed:12636367]
J Org Chem. 2003 Mar 21;68(6):2099-108.
A naturally occurring 1,1'-biphenanthrene, Blestriarene C (1), was prepared in 13 steps and 30% overall yield. The key steps are the ester-mediated nucleophilic aromatic substitution on 2,6-di-tert-butyl-4-methoxyphenyl 5-isopropoxy-2-methoxybenzoate (4) by 2-methoxy-4-methoxymethoxy-6-methylphenylmagnesium bromide (5) and a novel intramolecular cyclization of the resulting 4-isopropoxy-2'-methoxy-4'-methoxymethoxy-6'-methylbiphenyl-2-carboxylic ester 14 to 7-isopropoxy-4-methoxy-2-(methoxymethoxy)phenanthren-9-ol (15). The racemic Blestriarene C was optically resolved by chiral HPLC on a preparative scale to give several 10-mg yields of both the enantiomers in up to 95% ee. The absolute stereochemistry was determined to be S(a)-(-) by the axial chirality recognition method, which was based on the stereospecific formation of a 12-membered cyclic diester containing two biaryl-o,o'-diyl unites joined by ester -CO(2)- linkages. The validity of the method was confirmed by an X-ray crystallographic analysis and ab initio conformational analyses of such 12-membered cyclic diesters. It was found that Blestriarene C and its 7,7'-diisopropyl ether 2 underwent rapid photoracemization even under ambient light exposure.
First determination of the absolute stereochemistry of a naturally occurring 1,1'-biphenanthrene, (--)-blestriarene C, and its unexpected photoracemization.[Pubmed:12397993]
Chem Commun (Camb). 2002 Oct 7;(19):2234-5.
A naturally occurring 1,1'-biphenanthrene, Blestriarene C, was prepared and its absolute stereochemistry was determined to be Sa-(-) by an empirical method, during which the compound was found to undergo rapid photoracemization even under ambient light exposure.


