Angiotensin III (human, mouse)Aldosterone stimulator CAS# 13602-53-4 |

- Angiotensin III (human, mouse)
Catalog No.:BCC1031
CAS No.:13602-53-4
- Angiotensin 1/2 (1-9)
Catalog No.:BCC1005
CAS No.:34273-12-6
- Angiotensin I (human, mouse, rat)
Catalog No.:BCC1004
CAS No.:484-42-4
- Angiotensin 1/2 (1-5)
Catalog No.:BCC1035
CAS No.:58442-64-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 13602-53-4 | SDF | Download SDF |
| PubChem ID | 3082042 | Appearance | Powder |
| Formula | C46H66N12O9 | M.Wt | 931.09 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >93.1mg/ml in DMSO | ||
| Sequence | Arg-Val-Tyr-Ile-His-Pro-Phe | ||
| Chemical Name | (2S)-2-[[(2S)-1-[(2S)-2-[[(2S,3S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-5-(diaminomethylideneamino)pentanoyl]amino]-3-methylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-3-methylpentanoyl]amino]-3-(1H-imidazol-5-yl)propanoyl]pyrrolidine-2-carbonyl]amino]-3-phenylpropanoic acid | ||
| SMILES | CCC(C)C(C(=O)NC(CC1=CN=CN1)C(=O)N2CCCC2C(=O)NC(CC3=CC=CC=C3)C(=O)O)NC(=O)C(CC4=CC=C(C=C4)O)NC(=O)C(C(C)C)NC(=O)C(CCCN=C(N)N)N | ||
| Standard InChIKey | QMMRCKSBBNJCMR-KMZPNFOHSA-N | ||
| Standard InChI | InChI=1S/C46H66N12O9/c1-5-27(4)38(57-40(61)33(21-29-15-17-31(59)18-16-29)53-42(63)37(26(2)3)56-39(60)32(47)13-9-19-51-46(48)49)43(64)54-34(23-30-24-50-25-52-30)44(65)58-20-10-14-36(58)41(62)55-35(45(66)67)22-28-11-7-6-8-12-28/h6-8,11-12,15-18,24-27,32-38,59H,5,9-10,13-14,19-23,47H2,1-4H3,(H,50,52)(H,53,63)(H,54,64)(H,55,62)(H,56,60)(H,57,61)(H,66,67)(H4,48,49,51)/t27-,32-,33-,34-,35-,36-,37-,38-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous vasoconstrictor peptide, formed from the conversion of angiotensin II in vivo. Acts at both AT1 and AT2 receptors and increases vasopressin (anti-diuretic hormone) release and blood pressure. |

Angiotensin III (human, mouse) Dilution Calculator

Angiotensin III (human, mouse) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Angiotensin III (Asp | Arg-Val-Tyr-Ile-His-Pro-Phe) is a hexapeptide formed as a result of a cleavage at the N-terminus of Angiotensin II, a key factor in the Renin-Angiotensin-Aldosterone (RAAS) system by angiotensinases located in red blood cells and the vascular beds of most tissues. It has 40% of the presser activity of angiotensin II, but 100% of the aldosterone-producing activity.
In peripheral Ang systems, Ang II is the main effector peptide in the systemic circulation, although exogenous Ang III can be as potent as Ang II in, for example, stimulating aldosterone secretion [1] or inhibiting renin release [2]. In the rat brain, Ang III was found to be equipotent with Ang II as a pressor agent or dipsogen [3] and was bound as avidly to the nervous system as Ang II. Ang receptor subtype AT1 has greater affinity towards Ang II and is also responsive to Ang III, while the AT2 receptor subtype appears to be more sensitive to Ang III but less responsive to Ang II [4].
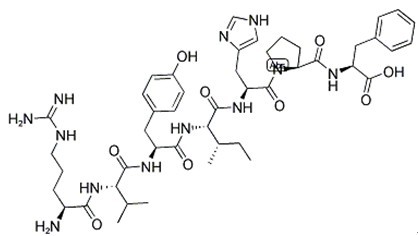
Fig. 1: Formula of Angiotensin III
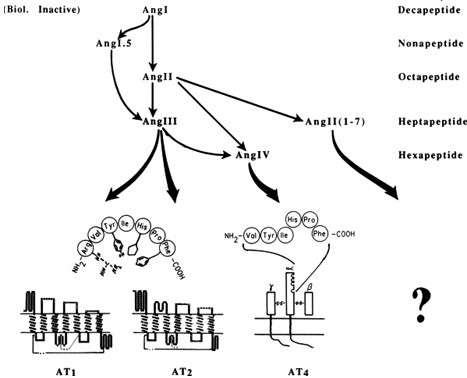
Fig. 2: Hypothesized interaction of each prominent brain angiotensin with its binding site.
Ref:
1. Blair-West, JR. et al. (1980) J. Endocrinol. 87, 409.
2. Fei, DTW. et al. (1980) Life Sci. 27, 1495.
3. Fink, GD. et al. (1985) Am. J. Physiol. Endocrinol. Metab. 249, E201.
4. Wright, JW. et al. (2011) Prog. Neurobiol. 95, 49.
- AVL-292 benzenesulfonate
Catalog No.:BCC1386
CAS No.:1360053-81-1
- Ranaconitine
Catalog No.:BCN3870
CAS No.:1360-76-5
- Tetracaine HCl
Catalog No.:BCC4399
CAS No.:136-47-0
- Phenazopyridine HCl
Catalog No.:BCC4698
CAS No.:136-40-3
- Caulophylline B
Catalog No.:BCN7499
CAS No.:1359978-55-4
- SR 2211
Catalog No.:BCC6310
CAS No.:1359164-11-6
- RP 67580
Catalog No.:BCC7134
CAS No.:135911-02-3
- Lupinol C
Catalog No.:BCN4809
CAS No.:135905-53-2
- H-D-Asp(OtBu)-OtBu.HCl
Catalog No.:BCC2898
CAS No.:135904-71-1
- Blumenol C glucoside
Catalog No.:BCN6189
CAS No.:135820-80-3
- erythro-Guaiacylglycerol beta-dihydroconiferyl ether
Catalog No.:BCN7025
CAS No.:135820-77-8
- Koumine
Catalog No.:BCN6190
CAS No.:1358-76-5
- (1R,2S)-1-Amino-2-indanol
Catalog No.:BCC8381
CAS No.:136030-00-7
- Fmoc-Tic-OH
Catalog No.:BCC3341
CAS No.:136030-33-6
- Schisanlactone E
Catalog No.:BCN2593
CAS No.:136040-43-2
- Fmoc-Cys(pMeBzl)-OH
Catalog No.:BCC3476
CAS No.:136050-67-4
- Justiciresinol
Catalog No.:BCN3419
CAS No.:136051-41-7
- [D-Lys3]-GHRP-6
Catalog No.:BCC5850
CAS No.:136054-22-3
- Desrhamnosylmartynoside
Catalog No.:BCN7648
CAS No.:136055-64-6
- 9-Dehydroxyeurotinone
Catalog No.:BCN7397
CAS No.:1360606-85-4
- Necrosulfonamide
Catalog No.:BCC7992
CAS No.:1360614-48-7
- 5-Methoxyisolariciresinol
Catalog No.:BCN7016
CAS No.:136082-41-2
- Onjixanthone II
Catalog No.:BCN7559
CAS No.:136083-93-7
- Lobetyolin
Catalog No.:BCN5894
CAS No.:136085-37-5
Osteoglycin prevents the development of age-related diastolic dysfunction during pressure overload by reducing cardiac fibrosis and inflammation.[Pubmed:28958774]
Matrix Biol. 2018 Mar;66:110-124.
The small leucine-rich proteoglycan osteoglycin has been implicated in matrix homeostasis in different organs, including the ischemic heart. However, whether osteoglycin modulates cardiac hypertrophy, fibrosis or inflammation in hypertensive heart disease and during aging remains unknown. Angiotensin-II-induced pressure overload increases cardiac osteoglycin expression, concomitant with the onset of inflammation and extracellular matrix deposition. Interestingly aging led to decreased cardiac levels of osteoglycin, yet absence of osteoglycin did not affect organ structure or cardiac function up to the age of 18months. However, Angiotensin-II infusion in combination with aging resulted in exaggerated cardiac fibrosis and inflammation in the osteoglycin null mice as compared to wild-type mice, resulting in increased diastolic dysfunction as determined by magnetic resonance imaging. In vitro, stimulation of bone marrow derived macrophages from osteoglycin null mice with Angiotensin-II resulted in significantly higher levels of ICAM-1 as well as pro-inflammatory cytokines and chemokines IL-1beta and MCP-1 as compared to WT cells. Further, stimulation of human cardiac fibroblasts with osteoglycin reduced cell proliferation and inhibited TGF-beta induced collagen gene expression. In mouse cardiac tissue, osteoglycin expression inversely correlated with TGF-beta expression and in cardiac biopsies of aortic stenosis patients, osteoglycin expression is significantly higher than in control biopsies. Interestingly, osteoglycin levels were higher in patients with less severe myocardial fibrosis and overall in the aortic stenosis patients osteoglycin levels negatively correlated with collagen content in the myocardium. In conclusion, osteoglycin expression is increased in the heart in response to pressure overload and its absence results in increased cardiac inflammation and fibrosis resulting in increased diastolic dysfunction.
S1PR1 (Sphingosine-1-Phosphate Receptor 1) Signaling Regulates Blood Flow and Pressure.[Pubmed:28607130]
Hypertension. 2017 Aug;70(2):426-434.
Nitric oxide is one of the major endothelial-derived vasoactive factors that regulate blood pressure (BP), and the bioactive lipid mediator S1P (sphingosine-1-phosphate) is a potent activator of endothelial nitric oxide synthase through G protein-coupled receptors. Endothelial-derived S1P and the autocrine/paracrine activation of S1PR (S1P receptors) play an important role in preserving vascular functions and BP homeostasis. Furthermore, FTY720 (fingolimod), binding to 4 out of 5 S1PRs recently approved by the Food and Drug Administration to treat autoimmune conditions, induces a modest and transient decrease in heart rate in both animals and humans, suggesting that drugs targeting sphingolipid signaling affect cardiovascular functions in vivo. However, the role of specific S1P receptors in BP homeostasis remains unknown. The aim of this study is to determine the role of the key vascular S1P receptors, namely, S1PR1 and S1PR3, in BP regulation in physiological and hypertensive conditions. The specific loss of endothelial S1PR1 decreases basal and stimulated endothelial-derived nitric oxide and resets BP to a higher-than-normal value. Interestingly, we identified a novel and important role for S1PR1 signaling in flow-mediated mechanotransduction. FTY720, acting as functional antagonist of S1PR1, markedly decreases endothelial S1PR1, increases BP in control mice, and exacerbates hypertension in angiotensin II mouse model, underlining the antihypertensive functions of S1PR1 signaling. Our study identifies S1P-S1PR1-nitric oxide signaling as a new regulatory pathway in vivo of vascular relaxation to flow and BP homeostasis, providing a novel therapeutic target for the treatment of hypertension.
Inhibition of MicroRNA-146a and Overexpression of Its Target Dihydrolipoyl Succinyltransferase Protect Against Pressure Overload-Induced Cardiac Hypertrophy and Dysfunction.[Pubmed:28611091]
Circulation. 2017 Aug 22;136(8):747-761.
BACKGROUND: Cardiovascular diseases remain the predominant cause of death worldwide, with the prevalence of heart failure continuing to increase. Despite increased knowledge of the metabolic alterations that occur in heart failure, novel therapies to treat the observed metabolic disturbances are still lacking. METHODS: Mice were subjected to pressure overload by means of angiotensin-II infusion or transversal aortic constriction. MicroRNA-146a was either genetically or pharmacologically knocked out or genetically overexpressed in cardiomyocytes. Furthermore, overexpression of dihydrolipoyl succinyltransferase (DLST) in the murine heart was performed by means of an adeno-associated virus. RESULTS: MicroRNA-146a was upregulated in whole heart tissue in multiple murine pressure overload models. Also, microRNA-146a levels were moderately increased in left ventricular biopsies of patients with aortic stenosis. Overexpression of microRNA-146a in cardiomyocytes provoked cardiac hypertrophy and left ventricular dysfunction in vivo, whereas genetic knockdown or pharmacological blockade of microRNA-146a blunted the hypertrophic response and attenuated cardiac dysfunction in vivo. Mechanistically, microRNA-146a reduced its target DLST-the E2 subcomponent of the alpha-ketoglutarate dehydrogenase complex, a rate-controlling tricarboxylic acid cycle enzyme. DLST protein levels significantly decreased on pressure overload in wild-type mice, paralleling a decreased oxidative metabolism, whereas DLST protein levels and hence oxidative metabolism were partially maintained in microRNA-146a knockout mice. Moreover, overexpression of DLST in wild-type mice protected against cardiac hypertrophy and dysfunction in vivo. CONCLUSIONS: Altogether we show that the microRNA-146a and its target DLST are important metabolic players in left ventricular dysfunction.
Involvement of arginine 878 together with Ca2+ in mouse aminopeptidase A substrate specificity for N-terminal acidic amino-acid residues.[Pubmed:28877217]
PLoS One. 2017 Sep 6;12(9):e0184237.
Aminopeptidase A (APA) is a membrane-bound zinc metalloprotease cleaving, in the brain, the N-terminal aspartyl residue of angiotensin II to generate angiotensin III, which exerts a tonic stimulatory effect on the control of blood pressure in hypertensive animals. Using a refined APA structure derived from the human APA crystal structure, we docked the specific and selective APA inhibitor, EC33 in the presence of Ca2+. We report the presence in the S1 subsite of Arg-887 (Arg-878 in mouse APA), the guanidinium moiety of which established an interaction with the electronegative sulfonate group of EC33. Mutagenic replacement of Arg-878 with an alanine or a lysine residue decreased the affinity of the recombinant enzymes for the acidic substrate, alpha-L-glutamyl-beta-naphthylamide, with a slight decrease in substrate hydrolysis velocity either with or without Ca2+. In the absence of Ca2+, the mutations modified the substrate specificity of APA for the acidic substrate, the mutated enzymes hydrolyzing more efficiently basic and neutral substrates, although the addition of Ca2+ partially restored the acidic substrate specificity. The analysis of the 3D models of the Arg-878 mutated APAs revealed a change in the volume of the S1 subsite, which may impair the binding and/or the optimal positioning of the substrate in the active site as well as its hydrolysis. These findings demonstrate the key role of Arg-878 together with Ca2 + in APA substrate specificity for N-terminal acidic amino acid residues by ensuring the optimal positioning of acidic substrates during catalysis.


