Angiotensin 1/2 (1-5)Vasoconstrictor CAS# 58442-64-1 |

- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- BMS-345541
Catalog No.:BCC1423
CAS No.:547757-23-3
- Bay 65-1942 free base
Catalog No.:BCC1408
CAS No.:600734-02-9
- Bay 65-1942 HCl salt
Catalog No.:BCC1409
CAS No.:600734-06-3
- Bay 65-1942 R form
Catalog No.:BCC1410
CAS No.:758683-21-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 58442-64-1 | SDF | Download SDF |
| PubChem ID | 71464373 | Appearance | Powder |
| Formula | C30H48N8O9 | M.Wt | 664.75 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >66.5mg/ml in DMSO | ||
| Sequence | H2N-Asp-Arg-Val-Tyr-Ile-OH | ||
| Chemical Name | (2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-3-carboxypropanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-3-methylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-3-methylpentanoic acid | ||
| SMILES | CCC(C)C(C(=O)O)NC(=O)C(CC1=CC=C(C=C1)O)NC(=O)C(C(C)C)NC(=O)C(CCCN=C(N)N)NC(=O)C(CC(=O)O)N | ||
| Standard InChIKey | UVPBVMCAVNABKX-JKTRTNGKSA-N | ||
| Standard InChI | InChI=1S/C30H48N8O9/c1-5-16(4)24(29(46)47)38-27(44)21(13-17-8-10-18(39)11-9-17)36-28(45)23(15(2)3)37-26(43)20(7-6-12-34-30(32)33)35-25(42)19(31)14-22(40)41/h8-11,15-16,19-21,23-24,39H,5-7,12-14,31H2,1-4H3,(H,35,42)(H,36,45)(H,37,43)(H,38,44)(H,40,41)(H,46,47)(H4,32,33,34)/t16?,19-,20-,21-,23-,24-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Angiotensin 1/2 (1-5) Dilution Calculator

Angiotensin 1/2 (1-5) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Angiotensin I/II (1-5) is a peptide (ASP-ARG-VAL-TYR-ILE) that contains the amino acids 1-5 and is converted from Angiotensin I/II.
Angiotensin I is formed by the action of renin on angiotensinogen, an α-2-globulin with 12 amino acids. Angiotensinogen is produced constitutively and released into the circulation mainly by the liver. Renin cleaves the peptide bond between the leucine (Leu) and valine (Val) residues on angiotensinogen, creating the ten-amino acid peptide angiotensin I. Angiotensin I is converted to angiotensin II (AII) through the removal of two C-terminal residues by angiotensin-converting enzyme (ACE), primarily by ACE within the lung.
Angiotensin is a peptide hormone that causes vasoconstriction and a subsequent increase in blood pressure. Angiotensin also stimulates the release of aldosterone, which promotes sodium retention in the distal nephron, thereby increasing blood pressure.
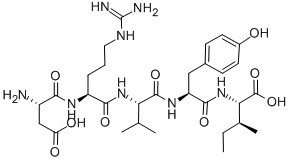
Figure1 Formula of Angiotensin I/II (1-5)
Ref:
1. Basso N, Terragno NA (December 2001). "History about the discovery of the renin-angiotensin system". Hypertension 38 (6): 1246–9.
2. Richard A. Preston. et. (1998). “Age-Race Subgroup Compared With Renin Profile as Predictors of Blood Pressure Response to Antihypertensive Therapy”. JAMA. 1998;280(13):1168-1172.
3. Williams GH, Dluhy RG (2008). "Chapter 336: Disorders of the Adrenal Cortex". In Loscalzo J, Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL. Harrison's principles of internal medicine. McGraw-Hill Medical.
- Boc-2-Nal-OH
Catalog No.:BCC3289
CAS No.:58438-04-3
- H-2-Nal-OH.HCl
Catalog No.:BCC3287
CAS No.:58438-03-2
- Dihydroresveratrol
Catalog No.:BCN5793
CAS No.:58436-28-5
- DL-Demethylcoclaurine
Catalog No.:BCC8317
CAS No.:5843-65-2
- Isopimaric acid
Catalog No.:BCN4618
CAS No.:5835-26-7
- 4,4'-Bis(N-carbazolyl)-1,1'-biphenyl
Catalog No.:BCC8660
CAS No.:58328-31-7
- Saikosaponin B3
Catalog No.:BCN8178
CAS No.:58316-42-0
- Saikosaponin B2
Catalog No.:BCN5916
CAS No.:58316-41-9
- Kusunokinin
Catalog No.:BCN3226
CAS No.:58311-20-9
- Nanaomycin C
Catalog No.:BCC4016
CAS No.:58286-55-8
- Kakkalide
Catalog No.:BCN8263
CAS No.:58274-56-9
- ICA 069673
Catalog No.:BCC7911
CAS No.:582323-16-8
- H-D-Leu-OMe.HCl
Catalog No.:BCC2681
CAS No.:5845-53-4
- N-(2-Hydroxy-4-methoxyphenyl)acetamide
Catalog No.:BCN1409
CAS No.:58469-06-0
- Darlingine
Catalog No.:BCN1906
CAS No.:58471-10-6
- Ferrugine
Catalog No.:BCN1910
CAS No.:58471-11-7
- Platycodin D
Catalog No.:BCN4982
CAS No.:58479-68-8
- Lemannine
Catalog No.:BCN3742
CAS No.:58480-54-9
- Olvanil
Catalog No.:BCC6855
CAS No.:58493-49-5
- ent-16beta,17-Isopropylidenedioxykaurane
Catalog No.:BCN1408
CAS No.:58493-71-3
- IOX 1
Catalog No.:BCC6192
CAS No.:5852-78-8
- Pseurotin A
Catalog No.:BCN7246
CAS No.:58523-30-1
- (-)-Cephaeline dihydrochloride
Catalog No.:BCN8323
CAS No.:5853-29-2
- Rebaudioside A
Catalog No.:BCN5900
CAS No.:58543-16-1
Post-spinal cord injury astrocyte-mediated functional recovery in rats after intraspinal injection of the recombinant adenoviral vectors Ad5-VEGF and Ad5-ANG.[Pubmed:28452633]
J Neurosurg Spine. 2017 Jul;27(1):105-115.
OBJECTIVE The most actively explored therapeutic strategy for overcoming spinal cord injury (SCI) is the delivery of genes encoding molecules that stimulate regeneration. In a mouse model of amyotrophic lateral sclerosis and in preliminary clinical trials in patients with amyotrophic lateral sclerosis, the combined administration of recombinant adenoviral vectors (Ad5-VEGF+Ad5-ANG) encoding the neurotrophic/angiogenic factors vascular endothelial growth factor ( VEGF) and angiogenin ( ANG) was found to slow the development of neurological deficits. These results suggest that there may be positive effects of this combination of genes in posttraumatic spinal cord regeneration. The objective of the present study was to determine the effects of Ad5-VEGF+Ad5-ANG combination therapy on motor function recovery and reactivity of astrocytes in a rat model of SCI. METHODS Spinal cord injury was induced in adult Wistar rats by the weight-drop method. Rats (n = 51) were divided into 2 groups: the experimental group (Ad5-VEGF+Ad5-ANG) and the control group (Ad5-GFP [green fluorescent protein]). Recovery of motor function was assessed using the Basso, Beattie, and Bresnahan scale. The duration and intensity of infectivity and gene expression from the injected vectors were assessed by immunofluorescent detection of GFP. Reactivity of glial cells was assessed by changes in the number of immunopositive cells expressing glial fibrillary acidic protein (GFAP), S100beta, aquaporin 4 (AQP4), oligodendrocyte transcription factor 2, and chondroitin sulfate proteoglycan 4. The level of S100beta mRNA expression in the spinal cord was estimated by real-time polymerase chain reaction. RESULTS Partial recovery of motor function was observed 30 days after surgery in both groups. However, Basso, Beattie, and Bresnahan scores were 35.9% higher in the Ad5-VEGF+Ad5-ANG group compared with the control group. Specific GFP signal was observed at distances of up to 5 mm in the rostral and caudal directions from the points of injection. A 1.5 to 2.0-fold increase in the number of GFAP(+), S100beta(+), and AQP4(+) cells was observed in the white and gray matter at a distance of up to 5 mm from the center of the lesion site in the caudal and rostral directions. At 30 days after injury, a 2-fold increase in S100beta transcripts was observed in the Ad5-VEGF+Ad5-ANG group compared with the control group. CONCLUSIONS Intraspinal injection of recombinant adenoviral vectors encoding VEGF and ANG stimulates functional recovery after traumatic SCI. The increased number of S100beta(+) astrocytes induced by this approach may be a beneficial factor for maintaining the survival and function of neurons. Therefore, gene therapy with Ad5-VEGF+Ad5-ANG vectors is an effective therapeutic method for SCI treatment.
Chronic overexpression of angiotensin-(1-7) in rats reduces cardiac reactivity to acute stress and dampens anxious behavior.[Pubmed:28288545]
Stress. 2017 Mar;20(2):189-196.
Angiotensin II (Ang II) acts as a pro-stress hormone, while other evidence indicates that angiotensin-(1-7) [Ang-(1-7)] attenuates physiological responses to emotional stress. To further test this hypothesis, in groups of 5-6 rats we evaluated autonomic, cardiovascular and behavioral parameters in male Sprague-Dawley (SD) and transgenic TGR(A1-7)3292 (TG) rats chronically overexpressing Ang-(1-7). Compared to SD rats, TG rats showed reduced baseline heart rate (HR; SD 380 +/- 16 versus TG 329 +/- 9 beats per minute (bpm), mean +/- standard error of mean, p < .05) and renal sympathetic discharge (SD 138 +/- 4 versus TG 117 +/- 5 spikes/second, p < .05). TG rats had an attenuated tachycardic response to acute air-puff stress (DeltaHR: SD 51 +/- 20 versus TG 1 +/- 3 bpm; p < .05), which was reversed by intracerebroventricular injection of the Mas receptor antagonist, A-779 (DeltaHR: SD 51 +/- 20 versus TG 63 +/- 15 bpm). TG rats showed less anxious behavior on the elevated plus maze, as revealed by more entries into open arms (SD 2 +/- 2 versus TG 47 +/- 5% relative to total entries; p < .05), and more time spent in the open arms (SD 5 +/- 4 versus TG 53 +/- 9% relative to total time, p < .05). By contrast with SD rats, diazepam (1.5 mg/kg, intraperitoneally) did not further reduce anxious behavior in TG rats, indicating a ceiling anxiolytic effect of Ang-(1-7) overexpression. Ang-(1-7) concentrations in hypothalamus and plasma, measured by mass spectrometry were two- and three-fold greater, respectively, in TG rats than in SD rats. Hence, increased endogenous Ang-(1-7) levels in TG rats diminishes renal sympathetic outflow and attenuates cardiac reactivity to emotional stress, which may be via central Mas receptors, and reduces anxious behavior. Lay summaryWe used a genetically modified rat model that produces above normal amounts of a peptide hormone called angiotensin-(1-7) to test whether this peptide can reduce some of the effects of stress. We found that angiotensin-(1-7), acting in the brain, can reduce anxiety and reduce the increase in heart rate associated with emotional stress. These findings may provide a lead for design of new drugs to reduce stress.
Angiotensin-(1-5), an active mediator of renin-angiotensin system, stimulates ANP secretion via Mas receptor.[Pubmed:27660028]
Peptides. 2016 Dec;86:33-41.
Angiotensin-(1-5) [Ang-(1-5)], which is a metabolite of Angiotensin-(1-7) [Ang-(1-7)] catalyzed by angiotensin-converting enzyme (ACE), is a pentapeptide of the renin-angiotensin system (RAS). It has been reported that Ang-(1-7) and Ang-(1-9) stimulate the secretion of atrial natriuretic peptide (ANP) via Mas receptor (Mas R) and Ang II type 2 receptor (AT2R), respectively. However, it still remains unknown whether Ang-(1-5) has a similar function to Ang-(1-7). We investigated the effect of Ang-(1-5) on ANP secretion and to define its signaling pathway using isolated perfused beating rat atria. Ang-(1-5) (0.3, 3, 10muM) stimulated high pacing frequency-induced ANP secretion in a dose-dependent manner. Ang-(1-5)-induced ANP secretion (3muM) was attenuated by the pretreatment with an antagonist of Mas R (A-779) but not by an antagonist of AT1R (losartan) or AT2R (PD123,319). An inhibitor for phosphatidylinositol 3-kinase (PI3K; wortmannin), protein kinase B (Akt; API-2), or nitric oxide synthase (NOS; L-NAME) also attenuated the augmentation of ANP secretion induced by Ang-(1-5). Ang-(1-5)-induced ANP secretion was markedly attenuated in isoproterenol-treated hypertrophied atria. The secretagogue effect of Ang-(1-5) on ANP secretion was similar to those induced by Ang-(1-9) and Ang-(1-7). These results suggest that Ang-(1-5) is an active mediator of renin-angiotensin system to stimulate ANP secretion via Mas R and PI3K-Akt-NOS pathway.
A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome.[Pubmed:28877748]
Crit Care. 2017 Sep 7;21(1):234.
BACKGROUND: Renin-angiotensin system (RAS) signaling and angiotensin-converting enzyme 2 (ACE2) have been implicated in the pathogenesis of acute respiratory distress syndrome (ARDS). We postulated that repleting ACE2 using GSK2586881, a recombinant form of human angiotensin-converting enzyme 2 (rhACE2), could attenuate acute lung injury. METHODS: We conducted a two-part phase II trial comprising an open-label intrapatient dose escalation and a randomized, double-blind, placebo-controlled phase in ten intensive care units in North America. Patients were between the ages of 18 and 80 years, had an American-European Consensus Criteria consensus diagnosis of ARDS, and had been mechanically ventilated for less than 72 h. In part A, open-label GSK2586881 was administered at doses from 0.1 mg/kg to 0.8 mg/kg to assess safety, pharmacokinetics, and pharmacodynamics. Following review of data from part A, a randomized, double-blind, placebo-controlled investigation of twice-daily doses of GSK2586881 (0.4 mg/kg) for 3 days was conducted (part B). Biomarkers, physiological assessments, and clinical endpoints were collected over the dosing period and during follow-up. RESULTS: Dose escalation in part A was well-tolerated without clinically significant hemodynamic changes. Part B was terminated after 39 of the planned 60 patients following a planned futility analysis. Angiotensin II levels decreased rapidly following infusion of GSK2586881, whereas angiotensin-(1-7) and angiotensin-(1-5) levels increased and remained elevated for 48 h. Surfactant protein D concentrations were increased, whereas there was a trend for a decrease in interleukin-6 concentrations in rhACE2-treated subjects compared with placebo. No significant differences were noted in ratio of partial pressure of arterial oxygen to fraction of inspired oxygen, oxygenation index, or Sequential Organ Failure Assessment score. CONCLUSIONS: GSK2586881 was well-tolerated in patients with ARDS, and the rapid modulation of RAS peptides suggests target engagement, although the study was not powered to detect changes in acute physiology or clinical outcomes. TRIAL REGISTRATION: ClinicalTrials.gov, NCT01597635 . Registered on 26 January 2012.
Reversal of Bone Marrow Mobilopathy and Enhanced Vascular Repair by Angiotensin-(1-7) in Diabetes.[Pubmed:27856608]
Diabetes. 2017 Feb;66(2):505-518.
The angiotensin (ANG)-(1-7)/Mas receptor (MasR) pathway activates vascular repair-relevant functions of bone marrow progenitor cells. We tested the effects of ANG-(1-7) on mobilization and vasoreparative functions of progenitor cells that are impaired in diabetes. The study was performed in streptozotocin-induced diabetic (db/db) mice. Diabetes resulted in a decreased number of Lineage(-)Sca-1(+)c-Kit(+) (LSK) cells in the circulation, which was normalized by ANG-(1-7). Diabetes-induced depletion of LSK cells in the bone marrow was reversed by ANG-(1-7). rho-Kinase (ROCK) activity was increased specifically in bone marrow LSK cells by ANG-(1-7) in diabetes, and the beneficial effects of ANG-(1-7) were prevented by fasudil. ANG-(1-7) increased Slit3 levels in the bone marrow supernatants, which activated ROCK in LSK cells and sensitized them for stromal-derived factor-1alpha (SDF)-induced migration. Diabetes prevented the mobilization of LSK cells in response to ischemia and impaired the recovery of blood flow, both of which were reversed by ANG-(1-7) in both models of diabetes. Genetic ablation of MasR prevented ischemia-induced mobilization of LSK cells and impaired blood flow recovery, which was associated with decreased proliferation and migration of LSK cells in response to SDF or vascular endothelial growth factor. These results suggest that MasR is a promising target for the treatment of diabetic bone marrow mobilopathy and vascular disease.


