KakkalideCAS# 58274-56-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 58274-56-9 | SDF | Download SDF |
| PubChem ID | 5490351 | Appearance | Powder |
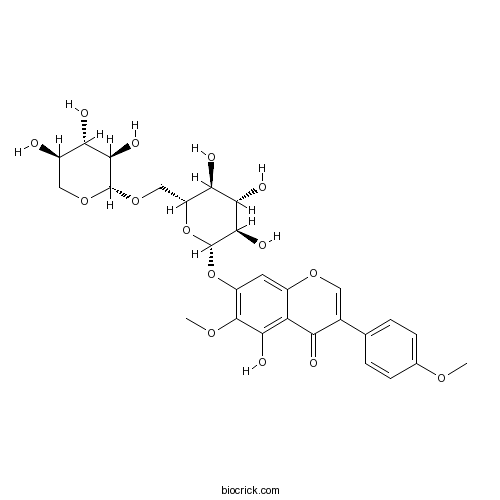
| Formula | C28H32O15 | M.Wt | 608.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-hydroxy-6-methoxy-3-(4-methoxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2S,3R,4S,5R)-3,4,5-trihydroxyoxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one | ||
| SMILES | COC1=CC=C(C=C1)C2=COC3=CC(=C(C(=C3C2=O)O)OC)OC4C(C(C(C(O4)COC5C(C(C(CO5)O)O)O)O)O)O | ||
| Standard InChIKey | QTVAYNGFFDZGDR-CIJVEFAYSA-N | ||
| Standard InChI | InChI=1S/C28H32O15/c1-37-12-5-3-11(4-6-12)13-8-39-15-7-16(26(38-2)22(33)18(15)19(13)30)42-28-25(36)23(34)21(32)17(43-28)10-41-27-24(35)20(31)14(29)9-40-27/h3-8,14,17,20-21,23-25,27-29,31-36H,9-10H2,1-2H3/t14-,17-,20+,21-,23+,24-,25-,27+,28-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Kakkalide is a potent lactate dehydrogenase (LDH) inhibitor. 2. Kakkalide attenuates ethanol-induced gastric injury in mice by inhibiting the infiltration of neutrophils. 3. Kakkalide can inhibit ROS-associated inflammation and ameliorated insulin-resistant endothelial dysfunction by beneficial effects on IRS-1 function. 4. Kakkalide has anti-inflammatory effects, it ameliorates carrageenan-induced inflammation in mice by inhibiting NF-κB pathway. 5. Kakkalide shows protective effects on ethanol-induced lethality and hepatic injury are dependent on its biotransformation by human intestinal microflora. |
| Targets | IL Receptor | NF-kB | ROS | Akt | PI3K | IkB | NO | PGE | COX | TNF-α | IKK |

Kakkalide Dilution Calculator

Kakkalide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6439 mL | 8.2196 mL | 16.4393 mL | 32.8785 mL | 41.0981 mL |
| 5 mM | 0.3288 mL | 1.6439 mL | 3.2879 mL | 6.5757 mL | 8.2196 mL |
| 10 mM | 0.1644 mL | 0.822 mL | 1.6439 mL | 3.2879 mL | 4.1098 mL |
| 50 mM | 0.0329 mL | 0.1644 mL | 0.3288 mL | 0.6576 mL | 0.822 mL |
| 100 mM | 0.0164 mL | 0.0822 mL | 0.1644 mL | 0.3288 mL | 0.411 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- ICA 069673
Catalog No.:BCC7911
CAS No.:582323-16-8
- BMS265246
Catalog No.:BCC3741
CAS No.:582315-72-8
- Betulin palmitate
Catalog No.:BCN5792
CAS No.:582315-55-7
- Tetraethyl ranelate
Catalog No.:BCC9177
CAS No.:58194-26-6
- H-DL-Ser-OMe.HCl
Catalog No.:BCC3100
CAS No.:5819-04-5
- Idebenone
Catalog No.:BCC4913
CAS No.:58186-27-9
- Ethyl 5-amino-4-cyano-3-(2-ethoxy-2-oxoethyl)thiophene-2-carboxylate
Catalog No.:BCC8975
CAS No.:58168-20-0
- Militarine
Catalog No.:BCN2551
CAS No.:58139-23-4
- NSC 207895 (XI-006)
Catalog No.:BCC2243
CAS No.:58131-57-0
- 2-Hydroxynaringenin
Catalog No.:BCN4820
CAS No.:58124-18-8
- SU 4312
Catalog No.:BCC7073
CAS No.:5812-07-7
- Aurantiamide
Catalog No.:BCN5790
CAS No.:58115-31-4
- Nanaomycin C
Catalog No.:BCC4016
CAS No.:58286-55-8
- Kusunokinin
Catalog No.:BCN3226
CAS No.:58311-20-9
- Saikosaponin B2
Catalog No.:BCN5916
CAS No.:58316-41-9
- Saikosaponin B3
Catalog No.:BCN8178
CAS No.:58316-42-0
- 4,4'-Bis(N-carbazolyl)-1,1'-biphenyl
Catalog No.:BCC8660
CAS No.:58328-31-7
- Isopimaric acid
Catalog No.:BCN4618
CAS No.:5835-26-7
- DL-Demethylcoclaurine
Catalog No.:BCC8317
CAS No.:5843-65-2
- Dihydroresveratrol
Catalog No.:BCN5793
CAS No.:58436-28-5
- H-2-Nal-OH.HCl
Catalog No.:BCC3287
CAS No.:58438-03-2
- Boc-2-Nal-OH
Catalog No.:BCC3289
CAS No.:58438-04-3
- Angiotensin 1/2 (1-5)
Catalog No.:BCC1035
CAS No.:58442-64-1
- H-D-Leu-OMe.HCl
Catalog No.:BCC2681
CAS No.:5845-53-4
Extraction and isolation of potential anti-stroke compounds from flowers of Pueraria lobata guided by in vitro PC12 cell model.[Pubmed:28236683]
J Chromatogr B Analyt Technol Biomed Life Sci. 2017 Mar 24;1048:111-120.
A simple and efficient method based on ultrafiltration liquid chromatography-mass spectrometry (UFLC-MS) was applied to rapidly screen and identify ligands for lactate dehydrogenase (LDH) from the flowers of Pueraria lobata, and the compounds were assessed for anti-stroke activity using a PC12 cell model. Seven major isoflavones, Kakkalide, 3'-hydroxy puerarin, puerarin, puerarin xyloside, tectoridin, tectorigenin, and ononin, were identified as potent LDH inhibitors. A continuous online method, which consisted of microwave-assisted extraction and countercurrent chromatography (MAE-CCC), was newly developed for scaled-up production of these compounds with high purity and efficiency. This novel approach, using UFLC-MS coupled with MAE-CCC and a PC12 cell model, provided a powerful tool for screening, extraction, and separation of LDH inhibitors from complex samples, and a useful platform for the large-scale production of functional food and nutraceutical ingredients.
Kakkalide and its metabolite irisolidone ameliorate carrageenan-induced inflammation in mice by inhibiting NF-kappaB pathway.[Pubmed:20686830]
Inflammation. 2011 Oct;34(5):344-51.
The anti-inflammatory activities of Kakkalide, a major constituent of the flower of Pueraria thunbergiana, and irisolidone, a metabolite of Kakkalide produced by intestinal microflora, against carrageenan-induced inflammation in air pouches on the backs of mice and in lipopolysaccharide (LPS)-stimulated peritoneal macrophages were investigated. Kakkalide and irisolidone down-regulated the gene expression of cytokines [tumor necrosis factor alpha (TNF-alpha) and interleukin-1 beta (IL-1beta)] and cyclooxygenase-2 (COX-2) and the production of pro-inflammatory cytokines, TNF-alpha and IL-1beta, and inflammatory mediators, NO and prostaglandin E(2) (PGE(2)), in LPS-stimulated peritoneal macrophages. These agents also inhibited the phosphorylation of IkappaB-alpha and the nuclear translocation of nuclear factor-kappa B (NF-kappaB). Orally administered Kakkalide and irisolidone significantly reduced carrageenan-induced inflammatory markers, leukocyte number, and protein amount in the exudates of the air pouch. These constituents also inhibited PGE(2) production and COX-2 inducible nitric oxide synthase, IL-1beta, and TNF-alpha expression. These agents also inhibited NF-kappaB activation. The anti-inflammatory effects of irisolidone were more potent than those of Kakkalide. Based on these findings, Kakkalide and irisolidone may inhibit inflammatory reactions via NF-kappaB pathway, and irisolidone, a metabolite of Kakkalide, may more potently inhibit these inflammatory reactions.
Irisolidone attenuates ethanol-induced gastric injury in mice by inhibiting the infiltration of neutrophils.[Pubmed:27546737]
Mol Nutr Food Res. 2017 Feb;61(2).
SCOPE: This study was designed to determine whether irisolidone and its glycoside Kakkalide, which are the major constituents of the flower of Pueraria lobata (Kudzu) can attenuate ethanol-induced gastritic injury in mice. METHODS AND RESULTS: Irisolidone and Kakkalide inhibited IL-8 secretion and NF-kappaB activation in lipopolysaccharide-stimulated KATO III cells. Therefore, we investigated their protective effects against ethanol-induced gastric injury in mice. Pretreatment with Kakkalide or irisolidone decreased the area of hemorrhagic ulcerative lesions caused by ethanol and suppressed stomach myeloperoxidase activity, CXCL4 secretion, and NF-kappaB activation. The ameliorating effect of irisolidone was more potent than that of Kakkalide. CONCLUSION: Irisolidone may attenuate ethanol-induced gastritis by inhibiting the infiltration of immune cells, particularly neutrophils, through the regulation of CXCL-4 or IL-8 secretion.
Kakkalide ameliorates endothelial insulin resistance by suppressing reactive oxygen species-associated inflammation.[Pubmed:23190749]
J Diabetes. 2013 Mar;5(1):13-24.
BACKGROUND: Kakkalide is the predominant isoflavone derived from the flowers of Pueraria lobata (Willd.) Ohwi. The aim of the present study was to investigate the effects of Kakkalide on insulin resistance in the endothelium. METHODS: Human umbilical vein endothelial cells (HUVEC) were stimulated with 100 mumol/L palmitate (PA) for 30 min and the effects of 30 min pretreatment with 0.1-10 mumol/L Kakkalide on reactive oxygen species (ROS)-associated inflammation in cells were evaluated by western blot analysis and reverse transcription-polymerase chain reaction. Furthermore, we investigated the biomodulation of insulin signaling by Kakkalide along the insulin receptor substrate (IRS)-1/Akt/endothelial nitric oxide synthase (eNOS) pathway. We also determined the effects of 30 min pretreatment with 0.1-10 mumol/L Kakkalide on insulin-mediated endothelium-dependent vasodilation of rat aorta in vitro following stimulation with 100 mumol/L PA. RESULTS: Kakkalide inhibited ROS overproduction and effectively restored mitochondrial membrane potential, demonstrating its chemoprotection of mitochondrial function. In addition, Kakkalide inhibited ROS-associated inflammation in the endothelium by inhibiting tumor necrosis factor-alpha and interleukin-6 production and gene expression, as well as suppressing the phosphorylation of c-Jun N-terminal kinase and IkappaB kinase beta/nuclear factor-kappaB. Inflammation impaired insulin phosphatidylinositol 3-kinase (PI3K) signaling and reduced insulin-mediated NO production in endothelial cells. Kakkalide facilitated PI3K signaling by positively regulating serine/tyrosine phosphorylation of IRS-1. CONCLUSIONS: Kakkalide inhibited ROS-associated inflammation and ameliorated insulin-resistant endothelial dysfunction by beneficial effects on IRS-1 function.
Protective effects of kakkalide from Flos puerariae on ethanol-induced lethality and hepatic injury are dependent on its biotransformation by human intestinal microflora.[Pubmed:14646251]
J Pharmacol Sci. 2003 Nov;93(3):331-6.
When Kakkalide, which was isolated from Flos Puerariae, was incubated with human fecal bacteria, Kakkalide was metabolized to irisolidone via kakkalidone. When Kakkalide (250 mg/kg) was orally administered to rats, irisolidone, but not Kakkalide, was detected in the blood. The mortality associated with ethanol treatment was slightly reduced when the mice were intraperitoneally treated with Kakkalide. Intraperitoneally administered Kakkalide and kakkalidone did not reduce alcohol toxicity. However, orally administered Kakkalide and intraperitoneally administered irisolidone significantly reduced the mortality. Orally administered Kakkalide and intraperitoneally injected irisolidone greatly reduced serum alanine aminotransferase and aspartate aminotransferase activities in ethanol-intoxified mice. Orally administered Kakkalide and intraperitoneally administered irisolidone significantly lowered the level of blood ethanol. The results indicate that Kakkalide is a prodrug of irisolidone in protecting against ethanol-induced lethality and hepatic injury.


