ICA 069673KV7.2/KV7.3 channel opener CAS# 582323-16-8 |
- CZC24832
Catalog No.:BCC1507
CAS No.:1159824-67-5
- PI3Kγ inhibitor 1
Catalog No.:BCC4180
CAS No.:1172118-03-4
- PI-103 Hydrochloride
Catalog No.:BCC1860
CAS No.:371935-79-4
- PIK-93
Catalog No.:BCC2519
CAS No.:593960-11-3
- TG100-115
Catalog No.:BCC1247
CAS No.:677297-51-7
- BKM120
Catalog No.:BCC1279
CAS No.:944396-07-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 582323-16-8 | SDF | Download SDF |
| PubChem ID | 10149311 | Appearance | Powder |
| Formula | C11H6ClF2N3O | M.Wt | 269.63 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 34 mg/mL (126.10 mM) *"≥" means soluble, but saturation unknown. | ||
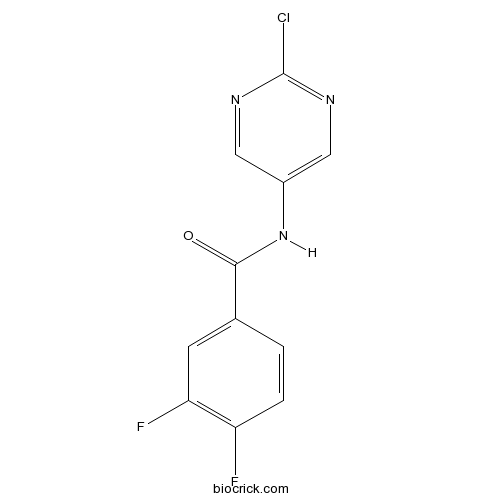
| Chemical Name | N-(2-chloropyrimidin-5-yl)-3,4-difluorobenzamide | ||
| SMILES | C1=CC(=C(C=C1C(=O)NC2=CN=C(N=C2)Cl)F)F | ||
| Standard InChIKey | IIBSHMFXVWTQSJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H6ClF2N3O/c12-11-15-4-7(5-16-11)17-10(18)6-1-2-8(13)9(14)3-6/h1-5H,(H,17,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective KV7.2/KV7.3 (KCNQ2/3) channel opener (EC50 = 0.69 μM). Exhibits 20-fold selectivity for KV7.2/KV7.3 over KV7.3/KV7.5, with no measurable activity over a panel of cardiac ion channels. Hyperpolarizes resting membrane potential and inhibits spontaneous action potentials in guinea pig detrusor muscle (DSM) isolated cells. Orally active in animal models of epilepsy. |

ICA 069673 Dilution Calculator

ICA 069673 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7088 mL | 18.5439 mL | 37.0879 mL | 74.1757 mL | 92.7197 mL |
| 5 mM | 0.7418 mL | 3.7088 mL | 7.4176 mL | 14.8351 mL | 18.5439 mL |
| 10 mM | 0.3709 mL | 1.8544 mL | 3.7088 mL | 7.4176 mL | 9.272 mL |
| 50 mM | 0.0742 mL | 0.3709 mL | 0.7418 mL | 1.4835 mL | 1.8544 mL |
| 100 mM | 0.0371 mL | 0.1854 mL | 0.3709 mL | 0.7418 mL | 0.9272 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- BMS265246
Catalog No.:BCC3741
CAS No.:582315-72-8
- Betulin palmitate
Catalog No.:BCN5792
CAS No.:582315-55-7
- Tetraethyl ranelate
Catalog No.:BCC9177
CAS No.:58194-26-6
- H-DL-Ser-OMe.HCl
Catalog No.:BCC3100
CAS No.:5819-04-5
- Idebenone
Catalog No.:BCC4913
CAS No.:58186-27-9
- Ethyl 5-amino-4-cyano-3-(2-ethoxy-2-oxoethyl)thiophene-2-carboxylate
Catalog No.:BCC8975
CAS No.:58168-20-0
- Militarine
Catalog No.:BCN2551
CAS No.:58139-23-4
- NSC 207895 (XI-006)
Catalog No.:BCC2243
CAS No.:58131-57-0
- 2-Hydroxynaringenin
Catalog No.:BCN4820
CAS No.:58124-18-8
- SU 4312
Catalog No.:BCC7073
CAS No.:5812-07-7
- Aurantiamide
Catalog No.:BCN5790
CAS No.:58115-31-4
- 1-(4-(3-Chloropropoxy)-3-methoxyphenyl)ethanone
Catalog No.:BCC8406
CAS No.:58113-30-7
- Kakkalide
Catalog No.:BCN8263
CAS No.:58274-56-9
- Nanaomycin C
Catalog No.:BCC4016
CAS No.:58286-55-8
- Kusunokinin
Catalog No.:BCN3226
CAS No.:58311-20-9
- Saikosaponin B2
Catalog No.:BCN5916
CAS No.:58316-41-9
- Saikosaponin B3
Catalog No.:BCN8178
CAS No.:58316-42-0
- 4,4'-Bis(N-carbazolyl)-1,1'-biphenyl
Catalog No.:BCC8660
CAS No.:58328-31-7
- Isopimaric acid
Catalog No.:BCN4618
CAS No.:5835-26-7
- DL-Demethylcoclaurine
Catalog No.:BCC8317
CAS No.:5843-65-2
- Dihydroresveratrol
Catalog No.:BCN5793
CAS No.:58436-28-5
- H-2-Nal-OH.HCl
Catalog No.:BCC3287
CAS No.:58438-03-2
- Boc-2-Nal-OH
Catalog No.:BCC3289
CAS No.:58438-04-3
- Angiotensin 1/2 (1-5)
Catalog No.:BCC1035
CAS No.:58442-64-1
Differential activation of vascular smooth muscle Kv7.4, Kv7.5, and Kv7.4/7.5 channels by ML213 and ICA-069673.[Pubmed:24944189]
Mol Pharmacol. 2014 Sep;86(3):330-41.
Recent research suggests that smooth muscle cells express Kv7.4 and Kv7.5 voltage-activated potassium channels, which contribute to maintenance of their resting membrane voltage. New pharmacologic activators of Kv7 channels, ML213 (N-mesitybicyclo[2.2.1]heptane-2-carboxamide) and ICA-069673 N-(6-chloropyridin-3-yl)-3,4-difluorobenzamide), have been reported to discriminate among channels formed from different Kv7 subtypes. We compared the effects of ML213 and ICA-069673 on homomeric human Kv7.4, Kv7.5, and heteromeric Kv7.4/7.5 channels exogenously expressed in A7r5 vascular smooth muscle cells. We found that, despite its previous description as a selective activator of Kv7.2 and Kv7.4, ML213 significantly increased the maximum conductance of homomeric Kv7.4 and Kv7.5, as well as heteromeric Kv7.4/7.5 channels, and induced a negative shift of their activation curves. Current deactivation rates decreased in the presence of the ML213 (10 muM) for all three channel combinations. Mutants of Kv7.4 (W242L) and Kv7.5 (W235L), previously found to be insensitive to another Kv7 channel activator, retigabine, were also insensitive to ML213 (10 muM). In contrast to ML213, ICA-069673 robustly activated Kv7.4 channels but was significantly less effective on homomeric Kv7.5 channels. Heteromeric Kv7.4/7.5 channels displayed intermediate responses to ICA-069673. In each case, ICA-069673 induced a negative shift of the activation curves without significantly increasing maximal conductance. Current deactivation rates decreased in the presence of ICA-069673 in a subunit-specific manner. Kv7.4 W242L responded to ICA-069673-like wild-type Kv7.4, but a Kv7.4 F143A mutant was much less sensitive to ICA-069673. Based on these results, ML213 and ICA-069673 likely bind to different sites and are differentially selective among Kv7.4, Kv7.5, and Kv7.4/7.5 channel subtypes.
The Novel KV7.2/KV7.3 Channel Opener ICA-069673 Reveals Subtype-Specific Functional Roles in Guinea Pig Detrusor Smooth Muscle Excitability and Contractility.[Pubmed:26087697]
J Pharmacol Exp Ther. 2015 Sep;354(3):290-301.
The physiologic roles of voltage-gated KV7 channel subtypes (KV7.1-KV7.5) in detrusor smooth muscle (DSM) are poorly understood. Here, we sought to elucidate the functional roles of KV7.2/KV7.3 channels in guinea pig DSM excitability and contractility using the novel KV7.2/KV7.3 channel activator ICA-069673 [N-(2-chloro-5-pyrimidinyl)-3,4-difluorobenzamide]. We employed a multilevel experimental approach using Western blot analysis, immunocytochemistry, isometric DSM tension recordings, fluorescence Ca(2+) imaging, and perforated whole-cell patch-clamp electrophysiology. Western blot experiments revealed the protein expression of KV7.2 and KV7.3 channel subunits in DSM tissue. In isolated DSM cells, immunocytochemistry with confocal microscopy further confirmed protein expression for KV7.2 and KV7.3 channel subunits, where they localize within the vicinity of the cell membrane. ICA-069673 inhibited spontaneous phasic, pharmacologically induced, and nerve-evoked contractions in DSM isolated strips in a concentration-dependent manner. The inhibitory effects of ICA-069673 on DSM spontaneous phasic and tonic contractions were abolished in the presence of the KV7 channel inhibitor XE991 [10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone dihydrochloride]. Under conditions of elevated extracellular K(+) (60 mM), the effects of ICA-069673 on DSM tonic contractions were significantly attenuated. ICA-069673 decreased the global intracellular Ca(2+) concentration in DSM cells, an effect blocked by the L-type Ca(2+) channel inhibitor nifedipine. ICA-069673 hyperpolarized the membrane potential and inhibited spontaneous action potentials of isolated DSM cells, effects that were blocked in the presence of XE991. In conclusion, using the novel KV7.2/KV7.3 channel activator ICA-069673, this study provides strong evidence for a critical role for the KV7.2- and KV7.3-containing channels in DSM function at both cellular and tissue levels.
N-Pyridyl and Pyrimidine Benzamides as KCNQ2/Q3 Potassium Channel Openers for the Treatment of Epilepsy.[Pubmed:24900334]
ACS Med Chem Lett. 2011 Mar 31;2(6):481-4.
A series of N-pyridyl benzamide KCNQ2/Q3 potassium channel openers were identified and found to be active in animal models of epilepsy and pain. The best compound 12 [ICA-027243, N-(6-chloro-pyridin-3-yl)-3,4-difluoro-benzamide] has an EC50 of 0.38 muM and is selective for KCNQ2/Q3 channels. This compound was active in several rodent models of epilepsy and pain but upon repeated dosing had a number of unacceptable toxicities that prevented further development. On the basis of the structure-activity relationships developed around 12, a second compound, 51, [N-(2-chloro-pyrimidin-5-yl)-3,4-difluoro-benzamide, ICA-069673], was prepared and advanced into a phase 1 clinical study. Herein, we describe the structure-activity relationships that led to the identification of compound 12 and to the corresponding pyrimidine 51.


