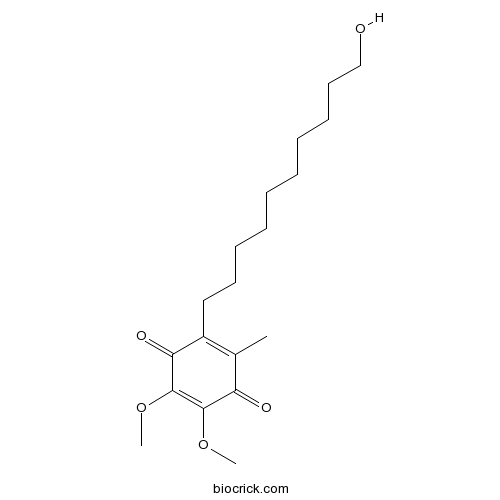
IdebenoneAntioxidant and neuroprotective agent CAS# 58186-27-9 |
- Gatifloxacin
Catalog No.:BCC1064
CAS No.:112811-59-3
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 58186-27-9 | SDF | Download SDF |
| PubChem ID | 3686 | Appearance | Powder |
| Formula | C19H30O5 | M.Wt | 338.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (295.47 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2-(10-hydroxydecyl)-5,6-dimethoxy-3-methylcyclohexa-2,5-diene-1,4-dione | ||
| SMILES | CC1=C(C(=O)C(=C(C1=O)OC)OC)CCCCCCCCCCO | ||
| Standard InChIKey | JGPMMRGNQUBGND-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H30O5/c1-14-15(12-10-8-6-4-5-7-9-11-13-20)17(22)19(24-3)18(23-2)16(14)21/h20H,4-13H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Antioxidant and neuroprotective agent. Protects mitochondrial membranes against lipid peroxidation and blocks glutamate neurotoxicity in vitro and in vivo. Inhibits apoptosis of astrocytes via increased NGF production. |

Idebenone Dilution Calculator

Idebenone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9547 mL | 14.7737 mL | 29.5473 mL | 59.0947 mL | 73.8683 mL |
| 5 mM | 0.5909 mL | 2.9547 mL | 5.9095 mL | 11.8189 mL | 14.7737 mL |
| 10 mM | 0.2955 mL | 1.4774 mL | 2.9547 mL | 5.9095 mL | 7.3868 mL |
| 50 mM | 0.0591 mL | 0.2955 mL | 0.5909 mL | 1.1819 mL | 1.4774 mL |
| 100 mM | 0.0295 mL | 0.1477 mL | 0.2955 mL | 0.5909 mL | 0.7387 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Idebenone
- Ethyl 5-amino-4-cyano-3-(2-ethoxy-2-oxoethyl)thiophene-2-carboxylate
Catalog No.:BCC8975
CAS No.:58168-20-0
- Militarine
Catalog No.:BCN2551
CAS No.:58139-23-4
- NSC 207895 (XI-006)
Catalog No.:BCC2243
CAS No.:58131-57-0
- 2-Hydroxynaringenin
Catalog No.:BCN4820
CAS No.:58124-18-8
- SU 4312
Catalog No.:BCC7073
CAS No.:5812-07-7
- Aurantiamide
Catalog No.:BCN5790
CAS No.:58115-31-4
- 1-(4-(3-Chloropropoxy)-3-methoxyphenyl)ethanone
Catalog No.:BCC8406
CAS No.:58113-30-7
- Fmoc-Arg(NO2)-OH
Catalog No.:BCC2596
CAS No.:58111-94-7
- Undulatoside A
Catalog No.:BCN6773
CAS No.:58108-99-9
- Isonicoteine
Catalog No.:BCN2152
CAS No.:581-50-0
- Anatabine
Catalog No.:BCN6899
CAS No.:581-49-7
- Suberosin
Catalog No.:BCN5791
CAS No.:581-31-7
- H-DL-Ser-OMe.HCl
Catalog No.:BCC3100
CAS No.:5819-04-5
- Tetraethyl ranelate
Catalog No.:BCC9177
CAS No.:58194-26-6
- Betulin palmitate
Catalog No.:BCN5792
CAS No.:582315-55-7
- BMS265246
Catalog No.:BCC3741
CAS No.:582315-72-8
- ICA 069673
Catalog No.:BCC7911
CAS No.:582323-16-8
- Kakkalide
Catalog No.:BCN8263
CAS No.:58274-56-9
- Nanaomycin C
Catalog No.:BCC4016
CAS No.:58286-55-8
- Kusunokinin
Catalog No.:BCN3226
CAS No.:58311-20-9
- Saikosaponin B2
Catalog No.:BCN5916
CAS No.:58316-41-9
- Saikosaponin B3
Catalog No.:BCN8178
CAS No.:58316-42-0
- 4,4'-Bis(N-carbazolyl)-1,1'-biphenyl
Catalog No.:BCC8660
CAS No.:58328-31-7
- Isopimaric acid
Catalog No.:BCN4618
CAS No.:5835-26-7
Evaluating the therapeutic potential of idebenone and related quinone analogues in Leber hereditary optic neuropathy.[Pubmed:28093355]
Mitochondrion. 2017 Sep;36:36-42.
Leber hereditary optic neuropathy (LHON) is an important cause of mitochondrial blindness among young adults. In this study, we investigated the potential of four quinone analogues (CoQ1, CoQ10, decylubiquinone and Idebenone) in compensating for the deleterious effect of the m.11778G>A mitochondrial DNA mutation. The LHON fibroblast cell lines tested exhibited reduced cell growth, impaired mitochondrial bioenergetics and elevated levels of reactive oxygen species (ROS). Idebenone increased ATP production and reduced ROS levels, but the effect was partial and cell-specific. The remaining quinone analogues had variable effects and a negative impact on certain mitochondrial parameters was observed in some cell lines.
Stability study of oral pediatric idebenone suspensions.[Pubmed:27583702]
Pharm Dev Technol. 2017 Mar;22(2):296-299.
Adapted forms for administration to infants are limited. The proposed study was performed to propose oral liquid formulations of Idebenone in Ora-Plus and either Ora-Sweet or Ora-Sweet SF, Ora-Blend, Ora-Blend SF and Inorpha. Each formulation was stored in 30 ml amber glass bottle at 5 or 25 degrees C for 90 days. Idebenone contents in these suspensions, determined by a stability-indicating high-performance liquid chromatography method, remained stable at least 90 days in Inorpha when stored at the two temperatures. In Ora-Blend, the stability was estimated at 14 days and in other suspensions at 20 days at the two temperatures. After 90 days storage, the pH of Ora-Plus and Ora-Sweet or Ora-Sweet SF changed between -0.10 and -0.25 units. For others suspensions, the pH changes were not significant (< -0.09 unit). No change was observed in color, odor or visual microbiology. To conclude, we recommended the use of Idebenone in Inorpha vehicle stable for at least 90 days at 25 degrees C.
New Micellar Electrokinetic Chromatographic Method for Analyzing Idebenone in Pediatric Formulations.[Pubmed:27881490]
J Chromatogr Sci. 2017 Mar 1;55(3):351-357.
A novel, simple and reliable method based on micellar electrokinetic chromatography with ultraviolet detection was developed to analyze Idebenone in a pediatric formulation. Idebenone is a synthetic short chain benzoquinone that acts as an electron carrier in the mitochondrial electron transport chain facilitating the production of adenosine triphosphate. It can be found in two different redox states that differ in their physiological properties. Idebenone has been investigated as a treatment in several neurological disorders like Friedreich's ataxia, Leber's hereditary optic neuropathy, mitochondrial encephalomyopathies and senile dementia. Accordingly, a micellar electrokinetic chromatography was employed to discriminate both redox forms. The final optimized system was validated in terms of selectivity, linearity (r2 0.992), limit of detection (0.5 microg/mL), limit of quantification (1.8 microg/mL), intra- and inter-day precision (RSD Idebenone in a pediatric formulation.
Treatment effect of idebenone on inspiratory function in patients with Duchenne muscular dystrophy.[Pubmed:27571420]
Pediatr Pulmonol. 2017 Apr;52(4):508-515.
Assessment of dynamic inspiratory function may provide valuable information about the degree and progression of pulmonary involvement in patients with Duchenne muscular dystrophy (DMD). The aims of this study were to characterize inspiratory function and to assess the efficacy of Idebenone on this pulmonary function outcome in a large and well-characterized cohort of 10-18 year-old DMD patients not taking glucocorticoid steroids (GCs) enrolled in the phase 3 randomized controlled DELOS trial. We evaluated the effect of Idebenone on the highest flow generated during an inspiratory FVC maneuver (maximum inspiratory flow; V'I,max(FVC)) and the ratio between the largest inspiratory flow during tidal breathing (tidal inspiratory flow; V'I,max(t)) and the V'I,max(FVC). The fraction of the maximum flow that is not used during tidal breathing has been termed inspiratory flow reserve (IFR). DMD patients in both treatment groups of DELOS (Idebenone, n = 31; placebo: n = 33) had comparable and abnormally low V'I,max(FVC) at baseline. During the study period, V'I,max(FVC) further declined by -0.29 L/sec in patients on placebo (95%CI: -0.51, -0.08; P = 0.008 at week 52), whereas it remained stable in patients on Idebenone (change from baseline to week 52: 0.01 L/sec; 95%CI: -0.22, 0.24; P = 0.950). The between-group difference favoring Idebenone was 0.27 L/sec (P = 0.043) at week 26 and 0.30 L/sec (P = 0.061) at week 52. In addition, during the study period, IFR improved by 2.8% in patients receiving Idebenone and worsened by -3.0% among patients on placebo (between-group difference 5.8% at week 52; P = 0.040). Although the clinical interpretation of these data is currently limited due to the scarcity of routine clinical practice experience with dynamic inspiratory function outcomes in DMD, these findings from a randomized controlled study nevertheless suggest that Idebenone preserved inspiratory muscle function as assessed by V'I,max(FVC) and IFR in patients with DMD. Pediatr Pulmonol. 2017;52:508-515. (c) 2016 The Authors. Pediatric Pulmonology Published by Wiley Periodicals, Inc.
CV-2619 protects cultured astrocytes against reperfusion injury via nerve growth factor production.[Pubmed:11040339]
Eur J Pharmacol. 2000 Oct 20;406(3):333-9.
In this study, we examined the effect of the neuroprotective agent 2, 3-dimethoxy-5-methyl-6-(10-hydroxydecyl)-1,4-benzoquinone (CV-2619) on reperfusion injury in cultured rat astrocytes after exposure to hydrogen peroxide (H(2)O(2))-containing medium. CV-2619 (10 nM to 10 microM) significantly attenuated the reperfusion-induced decrease in cell viability. The compound showed an anti-apoptotic effect in this astrocyte injury model. Antioxidants such as ascorbic acid, alpha-tocopherol and reduced glutathione also inhibited H(2)O(2) exposure-induced cytotoxicity. CV-2619 did not affect the levels of reactive oxygen species, but it increased nerve growth factor (NGF) production. The effect of CV-2619 on H(2)O(2) exposure-induced cytotoxicity was blocked by cycloheximide and anti-NGF antibody. The protective effect of CV-2619 was antagonized by the mitogen-activated protein (MAP)/extracellular signal-regulated kinase (ERK) kinase inhibitor 2'-amino-3'-methoxyflavone and the phosphatidylinositol-3 kinase inhibitor wortmannin. These findings suggest that the effect of CV-2619 is mediated at least partly by NGF production in astrocytes and that ERK and phosphatidylinositol-3 kinases play a role in the downstream mechanism.
Inhibitory effect of the neuroprotective agent idebenone on arachidonic acid metabolism in astrocytes.[Pubmed:10323265]
Eur J Pharmacol. 1999 Apr 9;370(2):161-7.
Idebenone, a compound with protective efficacy against neurotoxicity both in in vitro and in in vivo models, exists in two different oxidative states: the ubiquinol-derivative (reduced Idebenone) and the ubiquinone-derivative (oxidised Idebenone). In the present study, we have observed that both the redox forms of Idebenone have a dose-dependent inhibitory effect on the enzymatic metabolism of arachidonic acid in astroglial homogenates (IC50 reduced Idebenone: 1.76 +/- 0.86 microM; IC50 oxidised Idebenone: 16.65 +/- 3.48 microM), while in platelets, they are apparently less effective (IC50 reduced Idebenone: 18.28 +/- 4.70 microM; IC50 oxidised Idebenone: > 1 mM). We have also observed that the oxidised form preferentially inhibited cyclooxygenase vs. lipoxygenase metabolism (IC50 ratio lipoxygenase/cyclooxygenase: 3.22), while the reduced form did not discriminate between the two pathways (IC50 ratio lipoxygenase/cyclooxygenase: 1.38). In this respect, the inhibitory action of reduced Idebenone resembled that of the antioxidant nordihydroguaiaretic acid, while oxidised Idebenone behaved similarly as indomethacin and piroxicam--two typical anti-inflammatory agents. Our results suggest the existence of two distinct mechanisms of action for the two redox forms of Idebenone and a preferential action of the drug on arachidonic acid metabolism in the central nervous system.
Chemistry, toxicology, pharmacology and pharmacokinetics of idebenone: a review.[Pubmed:15374467]
Arch Gerontol Geriatr. 1990 Nov-Dec;11(3):177-86.
The name Idebenone (CV-2619) was given to a synthetic compound the chemical structure of which is 6-(10-hydroxydecyl)-2,3-dimethoxy-5-methyl-1,4-benzoquinone (commercial name in Japan: Avan). Although it is insoluble in water, suspensions of it can be applied and are absorbed relatively well from the intestine. Its acute toxicity is very low, and is well tolerated also in subacute and chronic treatments. It displayed no immunogenic or mutagenic side effects in the models studied so far. Pharmacological studies of Idebenone revealed that (i) it acts as a free radical scavenger and protects the mitochondrial membrane against lipid peroxidation; (ii) it prevents platelet aggregation; (iii) it improves vascular disorders due to strokes or experimental cerebral ischemia; (iv) it recovers the cholinergic and monoaminergic changes in the ischemic brain; (v) it improves overall brain function (including memory, also) in various animal models. Pharmacokinetic studies have confirmed that it reaches the brain cells even after per os administration. The main conclusion from the experimental data was that Idebenone may be of beneficial effect for the neurological disorders related to cerebral ischemia even in humans: the results obtained so far in human clinical trials confirmed the validity of this expectation for cases of human cerebrovascular disease.


