SuberosinCAS# 581-31-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 581-31-7 | SDF | Download SDF |
| PubChem ID | 68486 | Appearance | Cryst. |
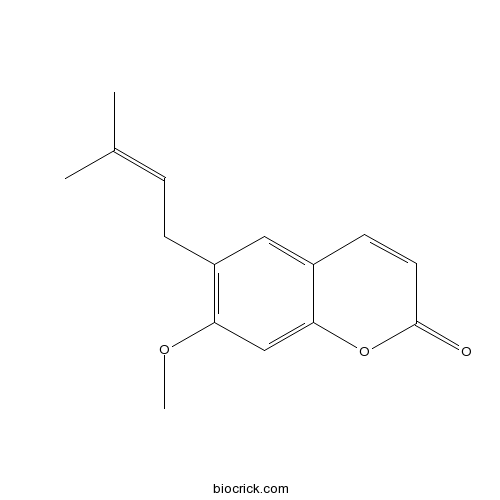
| Formula | C15H16O3 | M.Wt | 244.3 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 7-methoxy-6-(3-methylbut-2-enyl)chromen-2-one | ||
| SMILES | CC(=CCC1=C(C=C2C(=C1)C=CC(=O)O2)OC)C | ||
| Standard InChIKey | RSZDAYHEZSRVHS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H16O3/c1-10(2)4-5-11-8-12-6-7-15(16)18-14(12)9-13(11)17-3/h4,6-9H,5H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Suberosin exhibits anti-inflammatory, and anticoagulant activities, it also shows biting deterrent activity against Aedes aegypti, it may be useful for use as mosquito larvicidal agent. Suberosin inhibits PHA-induced PBMC proliferation, at least in part, through reduction of [Ca2+]i, ERK, NF-AT, and NF-kappaB activation, and early gene expression in PBMC including cyclins and cytokines, and arrest of cell cycle progression in the cells. |
| Targets | IFN-γ | IL Receptor | NF-kB | Calcium Channel | ERK |
| In vitro | Suberosin inhibits proliferation of human peripheral blood mononuclear cells through the modulation of the transcription factors NF-AT and NF-kappaB.[Pubmed: 17179947]Br J Pharmacol. 2007 Feb;150(3):298-312.Extracts of Plumbago zeylanica containing Suberosin exhibit anti-inflammatory activity. We purified Suberosin from such extracts and studied its effects on a set of key regulatory events in the proliferation of human peripheral blood mononuclear cells (PBMC) stimulated by phytohemagglutinin (PHA). Structure and in vitro antiparasitic activity of constituents of Citropsis articulata root bark.[Pubmed: 21985060]J Nat Prod. 2011 Oct 28;74(10):2286-9.From the results of an ethnomedicinal investigation of plants from Uganda with antimalarial activity, Citropsis articulata was selected because of the antiplasmodial effect of an ethyl acetate extract of its root bark. The identification of suberosin from prangos pabularia essential oil and its mosquito activity against aedes aegypti[Reference: WebLink]Rec. Nat. Prod. 10:3 (2016) 311-325.A detailed analysis of Prangos pabularia Lindl. (Apiaceae) fruit oil was performed by gas chromatography (GC-FID) and gas chromatography-mass spectrometry (GC-MS).
|
| Animal Research | Anticoagulant activity of isolated coumarins (suberosin and suberenol) and toxicity evaluation of Ferulago carduchorum in rats.[Pubmed: 25017518 ]Pharm Biol. 2014 Oct;52(10):1335-40Ferulago carduchorum Boiss. & Hausskn. (Apiaceae) is known as Chavil in Persian which grows in west of Iran. Local people add Chavil to dairy and oil ghee as a natural preservative to extend the expiration date.
The goal of this survey is the safety evaluation of the total extract of F. carduchorum in rats by determining both oral acute and subchronic toxicities; furthermore, the anticoagulant activity of isolated coumarins was evaluated.
|

Suberosin Dilution Calculator

Suberosin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0933 mL | 20.4666 mL | 40.9333 mL | 81.8666 mL | 102.3332 mL |
| 5 mM | 0.8187 mL | 4.0933 mL | 8.1867 mL | 16.3733 mL | 20.4666 mL |
| 10 mM | 0.4093 mL | 2.0467 mL | 4.0933 mL | 8.1867 mL | 10.2333 mL |
| 50 mM | 0.0819 mL | 0.4093 mL | 0.8187 mL | 1.6373 mL | 2.0467 mL |
| 100 mM | 0.0409 mL | 0.2047 mL | 0.4093 mL | 0.8187 mL | 1.0233 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- α-MSH
Catalog No.:BCC7420
CAS No.:581-05-5
- trans-3,4-Methylenedioxycinnamyl alcohol
Catalog No.:BCN1410
CAS No.:58095-76-4
- Miltefosine
Catalog No.:BCC4360
CAS No.:58066-85-6
- 24, 25-Dihydroxy VD2
Catalog No.:BCC1302
CAS No.:58050-55-8
- Averantin
Catalog No.:BCN7027
CAS No.:5803-62-3
- HOKU-81
Catalog No.:BCC1634
CAS No.:58020-43-2
- Epicorynoxidine
Catalog No.:BCN7554
CAS No.:58000-48-9
- Matairesinol
Catalog No.:BCN5789
CAS No.:580-72-3
- 2-Aminoquinoline
Catalog No.:BCC8555
CAS No.:580-22-3
- 3-Aminoquinoline
Catalog No.:BCC8620
CAS No.:580-17-6
- 6-Aminoquinoline
Catalog No.:BCC8766
CAS No.:580-15-4
- Uridine
Catalog No.:BCN4090
CAS No.:58-96-8
- Anatabine
Catalog No.:BCN6899
CAS No.:581-49-7
- Isonicoteine
Catalog No.:BCN2152
CAS No.:581-50-0
- Undulatoside A
Catalog No.:BCN6773
CAS No.:58108-99-9
- Fmoc-Arg(NO2)-OH
Catalog No.:BCC2596
CAS No.:58111-94-7
- 1-(4-(3-Chloropropoxy)-3-methoxyphenyl)ethanone
Catalog No.:BCC8406
CAS No.:58113-30-7
- Aurantiamide
Catalog No.:BCN5790
CAS No.:58115-31-4
- SU 4312
Catalog No.:BCC7073
CAS No.:5812-07-7
- 2-Hydroxynaringenin
Catalog No.:BCN4820
CAS No.:58124-18-8
- NSC 207895 (XI-006)
Catalog No.:BCC2243
CAS No.:58131-57-0
- Militarine
Catalog No.:BCN2551
CAS No.:58139-23-4
- Ethyl 5-amino-4-cyano-3-(2-ethoxy-2-oxoethyl)thiophene-2-carboxylate
Catalog No.:BCC8975
CAS No.:58168-20-0
- Idebenone
Catalog No.:BCC4913
CAS No.:58186-27-9
Suberosin inhibits proliferation of human peripheral blood mononuclear cells through the modulation of the transcription factors NF-AT and NF-kappaB.[Pubmed:17179947]
Br J Pharmacol. 2007 Feb;150(3):298-312.
BACKGROUND AND PURPOSE: Extracts of Plumbago zeylanica containing Suberosin exhibit anti-inflammatory activity. We purified Suberosin from such extracts and studied its effects on a set of key regulatory events in the proliferation of human peripheral blood mononuclear cells (PBMC) stimulated by phytohemagglutinin (PHA). EXPERIMENTAL APPROACH: Proliferation of PBMC in culture was measured by uptake of 3H-thymidine; production of cytokines and cyclins by Western blotting and RT-PCR. Transcription factors NF-AT and NF-kappaB were assayed by immunocytochemistry and EMSA. KEY RESULTS: Suberosin suppressed PHA-induced PBMC proliferation and arrested cell cycle progression from the G1 transition to the S phase. Suberosin suppressed, in activated PBMC, transcripts of interleukin-2 (IL-2), interferon-gamma (IFN-gamma), and cyclins D3, E, A, and B. DNA binding activity and nuclear translocation of NF-AT and NF-kappaB induced by PHA were blocked by Suberosin. Suberosin decreased the rise in intracellular Ca2+ concentration ([Ca2+]i) in PBMC stimulated with PHA. Suberosin did not affect phosphorylation of p38 and JNK but did reduce activation of ERK in PHA-treated PBMC. Pharmacological inhibitors of NF-kappaB, NF-AT, and ERK decreased expression of mRNA for the cyclins, IL-2, and IFN-gamma and cell proliferation in PBMC activated by PHA. CONCLUSIONS AND IMPLICATIONS: The inhibitory effects of Suberosin on PHA-induced PBMC proliferation, were mediated, at least in part, through reduction of [Ca2+]i, ERK, NF-AT, and NF-kappaB activation, and early gene expression in PBMC including cyclins and cytokines, and arrest of cell cycle progression in the cells. Our observations provide an explanation for the anti-inflammatory activity of P. zeylanica.
Structure and in vitro antiparasitic activity of constituents of Citropsis articulata root bark.[Pubmed:21985060]
J Nat Prod. 2011 Oct 28;74(10):2286-9.
From the results of an ethnomedicinal investigation of plants from Uganda with antimalarial activity, Citropsis articulata was selected because of the antiplasmodial effect of an ethyl acetate extract of its root bark. Thus, from the cyclohexane, ethyl acetate, and methanol extracts, two new heterocyclic compounds, omubioside (1) and katimborine (2), were isolated in addition to five known coumarins (rutarin (3), seselin (4), Suberosin (5), demethylSuberosin (6), and haploperoside (7)), two known alkaloids (5-hydroxynoracronycine (8) and 1,5-dihydroxy-2,3-dimethoxy-10-methyl-9-acridone (9)), trigonelline (10), and the limonoid 7alpha-obacunyl acetate (11). The best growth inhibitors of Plasmodium falciparum were alkaloids 8 and 9, with IC50 values of 0.9 and 3.0 mug/mL.
Anticoagulant activity of isolated coumarins (suberosin and suberenol) and toxicity evaluation of Ferulago carduchorum in rats.[Pubmed:25017518]
Pharm Biol. 2014 Oct;52(10):1335-40.
CONTEXT: Ferulago carduchorum Boiss. & Hausskn. (Apiaceae) is known as Chavil in Persian which grows in west of Iran. Local people add Chavil to dairy and oil ghee as a natural preservative to extend the expiration date. OBJECTIVE: The goal of this survey is the safety evaluation of the total extract of F. carduchorum in rats by determining both oral acute and subchronic toxicities; furthermore, the anticoagulant activity of isolated coumarins was evaluated. MATERIALS AND METHODS: The aerial parts of F. carduchorum were extracted by the percolation method. The anticoagulant activity of isolated coumarins was evaluated and the total extract was used to investigate acute and subchronic toxicity in rats. In the subchronic toxicity model, doses of 250, 500, and 1000 mg/kg of the extract were administered to treated groups for 30 consecutive days by gavage. RESULTS: According to the results of acute toxicity, the LD50 of Chavil extract was more than 2000 mg/kg. The subchronic study showed no significant difference (p > 0.05) between the groups treated with extract and control groups in hematological (erythrocyte, total and differential leukocyte, hematocrit, hemoglobin, platelet count) and biochemical parameter (glucose, albumin, cholesterol, triglycerides, aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase) evaluations. The isolated coumarins (Suberosin and suberenol) prolonged the prothrombin time (PT) at doses of 3 and 6 mg/kg compared with control (p < 0.05). The longest PT was for Suberosin at 6 mg/kg (17.4 s). CONCLUSION: In conclusion, oral administration of the Chavil extract did not cause either acute or subchronic toxicities although the coumarins showed anticoagulant effect in rats.


