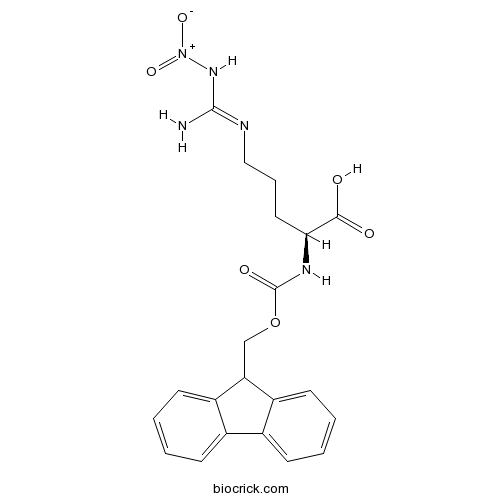
Fmoc-Arg(NO2)-OHCAS# 58111-94-7 |
- PF-4708671
Catalog No.:BCC5031
CAS No.:1255517-76-0
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- BI-D1870
Catalog No.:BCC5030
CAS No.:501437-28-1
- CMK
Catalog No.:BCC1489
CAS No.:821794-90-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 58111-94-7 | SDF | Download SDF |
| PubChem ID | 12096824 | Appearance | Powder |
| Formula | C21H23N5O6 | M.Wt | 441.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | C1=CC=C2C(=C1)C(C3=CC=CC=C32)COC(=O)NC(CCCN=C(N)N[N+](=O)[O-])C(=O)O | ||
| Standard InChIKey | RXMHIKWOZKQXCJ-SFHVURJKSA-N | ||
| Standard InChI | InChI=1S/C21H23N5O6/c22-20(25-26(30)31)23-11-5-10-18(19(27)28)24-21(29)32-12-17-15-8-3-1-6-13(15)14-7-2-4-9-16(14)17/h1-4,6-9,17-18H,5,10-12H2,(H,24,29)(H,27,28)(H3,22,23,25)/t18-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Fmoc-Arg(NO2)-OH Dilution Calculator

Fmoc-Arg(NO2)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2653 mL | 11.3266 mL | 22.6531 mL | 45.3063 mL | 56.6328 mL |
| 5 mM | 0.4531 mL | 2.2653 mL | 4.5306 mL | 9.0613 mL | 11.3266 mL |
| 10 mM | 0.2265 mL | 1.1327 mL | 2.2653 mL | 4.5306 mL | 5.6633 mL |
| 50 mM | 0.0453 mL | 0.2265 mL | 0.4531 mL | 0.9061 mL | 1.1327 mL |
| 100 mM | 0.0227 mL | 0.1133 mL | 0.2265 mL | 0.4531 mL | 0.5663 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fmoc-Arg(NO2)-OH
- Undulatoside A
Catalog No.:BCN6773
CAS No.:58108-99-9
- Isonicoteine
Catalog No.:BCN2152
CAS No.:581-50-0
- Anatabine
Catalog No.:BCN6899
CAS No.:581-49-7
- Suberosin
Catalog No.:BCN5791
CAS No.:581-31-7
- α-MSH
Catalog No.:BCC7420
CAS No.:581-05-5
- trans-3,4-Methylenedioxycinnamyl alcohol
Catalog No.:BCN1410
CAS No.:58095-76-4
- Miltefosine
Catalog No.:BCC4360
CAS No.:58066-85-6
- 24, 25-Dihydroxy VD2
Catalog No.:BCC1302
CAS No.:58050-55-8
- Averantin
Catalog No.:BCN7027
CAS No.:5803-62-3
- HOKU-81
Catalog No.:BCC1634
CAS No.:58020-43-2
- Epicorynoxidine
Catalog No.:BCN7554
CAS No.:58000-48-9
- Matairesinol
Catalog No.:BCN5789
CAS No.:580-72-3
- 1-(4-(3-Chloropropoxy)-3-methoxyphenyl)ethanone
Catalog No.:BCC8406
CAS No.:58113-30-7
- Aurantiamide
Catalog No.:BCN5790
CAS No.:58115-31-4
- SU 4312
Catalog No.:BCC7073
CAS No.:5812-07-7
- 2-Hydroxynaringenin
Catalog No.:BCN4820
CAS No.:58124-18-8
- NSC 207895 (XI-006)
Catalog No.:BCC2243
CAS No.:58131-57-0
- Militarine
Catalog No.:BCN2551
CAS No.:58139-23-4
- Ethyl 5-amino-4-cyano-3-(2-ethoxy-2-oxoethyl)thiophene-2-carboxylate
Catalog No.:BCC8975
CAS No.:58168-20-0
- Idebenone
Catalog No.:BCC4913
CAS No.:58186-27-9
- H-DL-Ser-OMe.HCl
Catalog No.:BCC3100
CAS No.:5819-04-5
- Tetraethyl ranelate
Catalog No.:BCC9177
CAS No.:58194-26-6
- Betulin palmitate
Catalog No.:BCN5792
CAS No.:582315-55-7
- BMS265246
Catalog No.:BCC3741
CAS No.:582315-72-8
Studies on lactam formation during coupling procedures of N alpha-N omega-protected arginine derivatives.[Pubmed:8738983]
Pept Res. 1996 Mar-Apr;9(2):88-91.
We evaluated the quantity of delta-lactam generated during the synthesis of arginine-containing dipeptides using Z-Arg(Tos)-OH, Boc-Arg(Tos)-OH, Fmoc-Arg(Boc)2-OH and Fmoc-Arg(Pmc)-OH and assayed several carboxyl-activating procedures for coupling the protected arginines to different amino components. We observed significant amounts of delta-lactam during the synthesis of Z-Arg(Tos)-methyl ester and Z-Arg(Tos)-amide, as well as of Boc-Arg(Tos)-chloromethyl ketone. The mixed anhydride coupling procedure and the di-Boc-protecting guanidino group induced more delta-lactam formation than any other coupling or NG-protection method. The amide, benzyl, 4-(NO2)-benzyl and methyl alpha-carboxyl-protected amino acids generated more delta-lactam than did those protected by tertbutyl or N2H2-Boc. So far it has not been possible to propose a general mechanism for delta-lactam formation or a process that completely abolishes it. Therefore, this side reaction should be considered almost inevitable. Its minimization requires examination of arginine-containing peptides in each specific synthesis.


