AnthraquinoneCAS# 84-65-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 84-65-1 | SDF | Download SDF |
| PubChem ID | 6780 | Appearance | Light yellow powder |
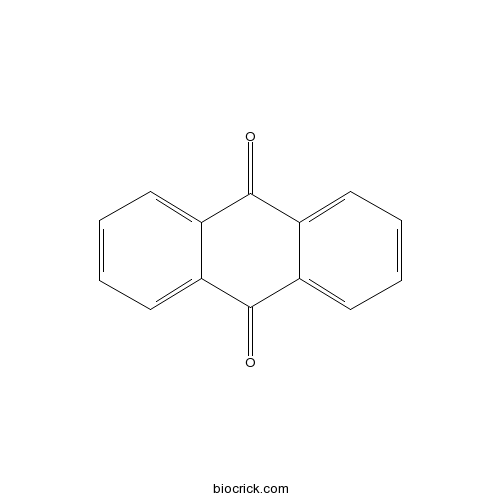
| Formula | C14H8O2 | M.Wt | 208 |
| Type of Compound | Quinones | Storage | Desiccate at -20°C |
| Solubility | Soluble in chloroform; practically insoluble in water | ||
| Chemical Name | anthracene-9,10-dione | ||
| SMILES | C1=CC=C2C(=C1)C(=O)C3=CC=CC=C3C2=O | ||
| Standard InChIKey | RZVHIXYEVGDQDX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H8O2/c15-13-9-5-1-2-6-10(9)14(16)12-8-4-3-7-11(12)13/h1-8H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Anthraquinone Dilution Calculator

Anthraquinone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8077 mL | 24.0385 mL | 48.0769 mL | 96.1538 mL | 120.1923 mL |
| 5 mM | 0.9615 mL | 4.8077 mL | 9.6154 mL | 19.2308 mL | 24.0385 mL |
| 10 mM | 0.4808 mL | 2.4038 mL | 4.8077 mL | 9.6154 mL | 12.0192 mL |
| 50 mM | 0.0962 mL | 0.4808 mL | 0.9615 mL | 1.9231 mL | 2.4038 mL |
| 100 mM | 0.0481 mL | 0.2404 mL | 0.4808 mL | 0.9615 mL | 1.2019 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Anthraflavic acid
Catalog No.:BCC8831
CAS No.:84-60-6
- Tectoquinone
Catalog No.:BCN3481
CAS No.:84-54-8
- Stylopine
Catalog No.:BCN3715
CAS No.:84-39-9
- Syrosingopine
Catalog No.:BCN5365
CAS No.:84-36-6
- Ophiohayatone C
Catalog No.:BCN3608
CAS No.:84-33-3
- Rutaecarpine
Catalog No.:BCN4385
CAS No.:84-26-4
- Hexestrol
Catalog No.:BCC4484
CAS No.:84-16-2
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
- 2,7-Dimethyl-1,4-dihydroxynaphthalene 1-O-glucoside
Catalog No.:BCN7611
CAS No.:839711-70-5
- Pluripotin
Catalog No.:BCC6178
CAS No.:839707-37-8
- GNF-7
Catalog No.:BCC6529
CAS No.:839706-07-9
- 4-Epicommunic acid
Catalog No.:BCN4382
CAS No.:83945-57-7
- Diisobutyl phthalate
Catalog No.:BCN7148
CAS No.:84-69-5
- Dibutyl Phthalate
Catalog No.:BCC8411
CAS No.:84-74-2
- Lapachol
Catalog No.:BCN4391
CAS No.:84-79-7
- Vitamin K1
Catalog No.:BCN2209
CAS No.:84-80-0
- Xanthoxyletin
Catalog No.:BCN6579
CAS No.:84-99-1
- Fmoc-N-Me-Ala-OH
Catalog No.:BCC3210
CAS No.:84000-07-7
- Fmoc-N-Me-Val-OH
Catalog No.:BCC3358
CAS No.:84000-11-3
- Helioxanthin 8-1
Catalog No.:BCC5415
CAS No.:840529-13-7
- Lamotrigine
Catalog No.:BCC5051
CAS No.:84057-84-1
- Roquinimex
Catalog No.:BCC5355
CAS No.:84088-42-6
- 1-Benzhydrylpiperazine
Catalog No.:BCC8453
CAS No.:841-77-0
- Wilforlide A
Catalog No.:BCN4383
CAS No.:84104-71-2
[Qualitative analysis of compositions of anthraquinone series working solution by gas chromatography-mass spectrometry].[Pubmed:30977347]
Se Pu. 2019 Apr 8;37(4):432-437.
A method for the qualitative analysis of compositions of Anthraquinone working solution (WS)/hydrogenated working solution (HWS) by gas chromatography-mass spectrometry (GC-MS) was developed. The composition of ethylAnthraquinone (eAQ) WS/HWS was identified by GC-MS. Then the samples of amylAnthraquinone (AAQ) WS/HWS were analyzed by GC-MS. Combined with the reaction mechanism, the composition of AAQ WS/HWS was inferred. The list of the degradation as well as the intermediate products in the industrial synthesis of H2O2 in Anthraquinone WS was generated, and the information obtained regarding the composition of the Anthraquinone WS/HWS was helpful in identifying and removing the unacceptable degradation products.
Emodin, a natural anthraquinone, suppresses liver cancer in vitro and in vivo by regulating VEGFR2 and miR-34a.[Pubmed:30976957]
Invest New Drugs. 2019 Apr 11. pii: 10.1007/s10637-019-00777-5.
The pharmacokinetic (PK) and potential effects of Emodin on liver cancer were systematically evaluated in this study. Both the intragastric administration (i.g.) and hypodermic injection (i.h.) of Emodin exhibited a strong absorption (absorption rate < 1 h) and elimination capacity (t1/2 approximately 2 h). The tissue distribution of Emodin after i.h. was rapid and wide. The stability of Emodin in three species of liver microsomes wasrat >human> beagle dog. These PK data provided the basis for the subsequent animal experiments. In liver cancer patient tissues, the expression of vascular endothelial growth factor (VEGF)-induced signaling pathways, including phosphorylated VEGF receptor 2 (VEGFR2), AKT, and ERK1/2,were simultaneously elevated, but miR-34a expression was reduced and negatively correlated with SMAD2 and SMAD4. Emodin inhibited the expression of SMAD2/4 in HepG2 cells by inducing the miR-34a level. Subsequently, BALB/c nude mice received a daily subcutaneous injection of HepG2 cells with or without Emodin treatment (1 mg/kg or 10 mg/kg), and Emodin inhibited tumorigenesis and reduced the mortality rate in a dose-dependent manner. In vivo experiments showed that cell proliferation, migration, and invasion were promoted by VEGF or miR-34a signal treatment but were inhibited when combined with Emodin treatment. All these results demonstrated that Emodin inhibited tumorigenesis in liver cancer by simultaneously inhibiting the VEGFR2-AKT-ERK1/2signaling pathway and promoting a miR-34a-mediated signaling pathway.
Quantitative structure-retention relationship for chromatographic behaviour of anthraquinone derivatives through considering organic modifier features in micellar liquid chromatography.[Pubmed:30975530]
J Chromatogr A. 2019 Mar 30. pii: S0021-9673(19)30347-4.
A simple and informative quantitative structure-retention relationship (QSRR) model has been introduced for prediction of retention times in some Anthraquinone derivatives using reversed-phase micellar liquid chromatography (MLC) technique. In the developed multiple linear regression model, the structural descriptors of analytes as well as the empirical parameters of organic modifiers in the applied MLC systems have been considered. Retention times of 77 chromatographic samples (16 Anthraquinones were evaluated by using 6 different organic modifiers) were experimentally determined and utilized as the independent variables of the QSRR model. Five small-chain alcohols (methanol, ethanol, propanol, butanol, and pentanol) as well as acetonitrile were used as the eluent modifiers. A five-parametric model was attained for the logarithm of the retention time values which covered about 96 and 95% variance of the chromatographic data in training and cross-validation, respectively. The presence of an excellent correlation coefficient for external validation test (= 0.94) and a well-applicable domain proved the prediction ability of the constructed model. Both validity and reliability of the formulated model were examined through its application on diverse random-selected training and test sets. Moreover, quantum chemical calculations were performed in the framework of density functional theory to simulate the interactions between AQs and modifiers and gain mechanistic details about the retention behavior in the MLC system.
Improved degradation of anthraquinone dye by electrochemical activation of PDS.[Pubmed:30974246]
Ecotoxicol Environ Saf. 2019 Apr 8;177:77-85.
Electrochemical oxidation (EO) coupled with peroxydisulfate (PDS) activation as a synergistic wastewater treatment process (PDS/EO) was performed to degrade Anthraquinone dye-Reactive Brilliant Blue (RBB) in aqueous solution. Introducing PDS into the EO improved the RBB removal than the sole PDS and conventional EO systems. The RBB could activate PDS to a certain degree by itself. By the comparison of various inorganic ions addition, it showed that adding NO3(-) as the background electrolyte was more effective than the systems using the Cl(-) and SO4(2-), respectively. In this PDS/EO-NO3(-) system, increasing PDS concentration (1-5mmolL(-1)) and current density (5-10mAcm(-2)) considerably promoted the degradation of RBB. The adjustment of the solution pH displayed that the acidic and neutral condition was beneficial to the RBB removal, and the synergistic effect was inverse ratio to the RBB initial concentration. Furthermore, the scavenger experiments verified that both SO4(.-) and HO. were the major active substances in the RBB decomposition, and other reactive oxygen species also had considerable contributions. Thereinto NO3(-) only act a catalytic agent to improve the generation of active matters in the PDS/EO-NO3(-). Overall, the proposed synergistic process could serve as an efficient method for the degradation of Anthraquinone dye.
Layer-by-Layer Assembly and Electrochemical Study of Alizarin Red S-Based Thin Films.[Pubmed:30960149]
Polymers (Basel). 2019 Jan 18;11(1). pii: polym11010165.
Electroactive organic dyes incorporated in layer-by-layer (LbL) assemblies are of great interest for a variety of applications. In this paper, Alizarin Red S (ARS), an electroactive Anthraquinone dye, is employed to construct LbL (BPEI/ARS)n films with branched poly(ethylene imine) (BPEI) as the complementary polymer. Unconventional LbL methods, including co-adsorption of ARS and poly(4-styrene sulfonate) (PSS) with BPEI to assemble (BPEI/(ARS+PSS))n, as well as pre-complexation of ARS with BPEI and further assembly with PSS to fabricate ((BPEI+ARS)/PSS)n, are designed for investigation and comparison. Film growth patterns, UV(-)Vis spectra and surface morphology of the three types of LbL assemblies are measured and compared to reveal the formation mechanism of the LbL films. Electrochemical properties including cyclic voltammetry and spectroelectrochemistry of (BPEI/ARS)120, (BPEI/(ARS+PSS))120 and ((BPEI+ARS)/PSS)120 films are studied, and the results show a slight color change due to the redox reaction of ARS. ((BPEI+ARS)/PSS)120 shows the best stability among the three samples. It is concluded that the manner of dye- incorporation has a great effect on the electrochemical properties of the resultant films.
Human exposure to banned pesticides reported to the French Poison Control Centers: 2012-2016.[Pubmed:30953934]
Environ Toxicol Pharmacol. 2019 Mar 30;69:51-56.
In 2008, 30 active substances from plant protection products were banned from marketing in France. Nevertheless, the French Poison Control Centers continue to see cases of poisoning caused by these active substances that are no longer approved. The aim of this study was to describe the characteristics of the reported cases in mainland France and in overseas French territories, over the period 2012-2016. A total of 408 cases of human exposure were reported during the study period. The most commonly reported substances were dichlorvos (24.8%, n = 108), paraquat (23.8%, n = 97), aldicarb (14.7%, n = 60), diuron (9.6%, n = 39), dinocap (5.1%, n = 21), methomyl (4.2%, n = 17), carbofuran (3.9%, n = 16), Anthraquinone (2.9%, n = 12) and carbendazim (2.7%, n = 11). The number of cases of intoxication dropped sharply between 2012 (n = 119) and 2016 (n = 47), except in the overseas French territories. Among the 72 serious cases (severe or life-threatening or with a fatal outcome), the most common substances involved were paraquat (n = 34), aldicarb (n = 24) and carbofuran (n = 7). This study suggests persistent use of carbamate insecticides, the existence of illegal imports of dichlorvos or paraquat-based products, and the use of certain banned fungicides in the professional agricultural sector. Information and collection campaigns are therefore essential after the withdrawal of marketing authorization for the plant protection products.
Extending the Lifetime of Organic Flow Batteries via Redox State Management.[Pubmed:30945536]
J Am Chem Soc. 2019 Apr 4.
Redox flow batteries based on quinone-bearing aqueous electrolytes have emerged as promising systems for energy storage from intermittent renewable sources. The lifetime of these batteries is limited by quinone stability. Here, we confirm that 2,6-dihydroxyanthrahydroquinone (DHAHQ) tends to form an anthrone intermediate that is vulnerable to subsequent irreversible dimerization. We demonstrate quantitatively that this decomposition pathway is responsible for the loss of battery capacity. Computational studies indicate that the driving force for anthrone formation is greater for Anthraquinones with lower reduction potentials. We show that the decomposition can be substantially mitigated. We demonstrate that conditions minimizing anthrone formation and avoiding anthrone dimerization slow the capacity loss rate by over an order of magnitude. We anticipate that this mitigation strategy readily extends to other Anthraquinone-based flow batteries and is thus an important step toward realizing renewable electricity storage through long-lived organic flow batteries.
Herbal preparations use in prevention and treatment of gastrointestinal and hepatic disorders-Data from Vojvodina, Serbia.[Pubmed:30935541]
Complement Ther Med. 2019 Apr;43:265-270.
OBJECTIVE: Gastrointestinal (GI) disorders are estimated to be frequent among general population. Various types of traditional and complementary therapies, primarily phytotherapy, can be used for prevention and treatment of many diseases and conditions, including GI complaints. Thus, the aim of this study was to investigate the patterns of use of medicinal herbs in treatment and prevention of GI disorders, together with their efficacy and safety. METHODS: A prospective, repeated cross-sectional, descriptive study was conducted in the form of a specifically created questionnaire, filled in by consumers and/or patients in pharmacies on the territory of Autonomous Province of Vojvodina, Republic of Serbia. All data were statistically analyzed in Microsoft Excel 2007. RESULTS: In the total number of 1137 patients, 10.4% declared themselves as consumers of phytopreparations for GI disorders. The most common diseases were constipation (44%) and dyspepsia (23%). The most frequently used preparations contained laxatives (with both Anthraquinones and dietary fibers), artichoke and silymarin. Iberogast(R) was also frequently used. Pharmacists were the main source of recommendation for the most adequate herbal remedies. At the same time, phytopreparations were well tolerated, with no major side effects, and were evidently or presumably effective. CONCLUSIONS: Some mild and moderate GI disorders seem to be treated frequently with phytopreparatons. Various herbal remedies are well accepted by patients, and the phytopreparations seem to have favorable ratio of safety and efficacy. Further integration into conventional medicine will improve the quality of the products used and provide a rational plan of use of medicinal plants.
Modulating electronic coupling at the quantum dot/molecule interface by wavefunction engineering.[Pubmed:30927884]
J Chem Phys. 2019 Mar 28;150(12):124704.
In this work, we use wavefunction engineering by varying the size of Quantum Dots (QDs) and tuning the delocalization (or diffuseness) of frontier orbitals of an acceptor molecule to modulate charge transfer dynamics at the QD/molecule interface. For this purpose, we apply our recently developed bulk-adjusted linear combination of atomic orbitals (BA-LCAO) approach for nanostructures and a density functional theory (DFT) for the acceptor molecules. These electronic structure calculations, combined with extensive molecular dynamics simulations using a fragmented molecular mechanics (FraMM) force field, reveal intimate details of charge transfer across the QD/Acceptor interface. For the spherical wurtzite-(CdSe)201 and (CdSe)693 nanostructures, as model QDs with respective 2.8 and 4.1 nm diameters, and Anthraquinone-2,3-dicarboxylic acid and its derivatives with the 7-OH, 7-OF, 10-BH, and 10-CH2 substituents, as model molecular acceptors, we find that (1) both the electron donating and withdrawing groups greatly enhance hole transfer by means of diffusing the acceptor HOMO; (2) electron transfer is affected only by the electron donating groups; (3) solvent effects are largely negligible for the orbital overlaps, and (4) consistent with spatial confinement theories, the electron density of the smaller QD penetrates farther into the vacuum than the corresponding density of the larger QD leading to stronger coupling with the acceptor. These findings suggest that (a) one can effectively control charge transfer across the QD/molecule interface by either changing the size of the QD or by tuning diffuseness of frontier orbitals of the acceptor molecule and (b) the combination of the recently developed BA-LCAO approach for QDs with a DFT for the acceptor molecules, facilitated by the use of the FraMM force field and extensive molecular dynamics simulations, provide qualitatively accurate description of charge transfer dynamics at the QD/acceptor interface.
Liquid Phase and Microwave-Assisted Extractions for Multicomponent Phenolic Pattern Determination of Five Romanian Galium Species Coupled with Bioassays.[Pubmed:30925810]
Molecules. 2019 Mar 28;24(7). pii: molecules24071226.
Background: Galium is a plant rich in iridoid glycosides, flavonoids, Anthraquinones, and small amounts of essential oils and vitamin C. Recent works showed the antibacterial, antifungal, antiparasitic, and antioxidant activity of this plant genus. Methods: For the determination of the multicomponent phenolic pattern, liquid phase microextraction procedures were applied, combined with HPLC-PDA instrument configuration in five Galium species aerial parts (G. verum, G. album, G. rivale, G. pseudoaristatum, and G. purpureum). Dispersive Liquid(-)Liquid MicroExtraction (DLLME) with NaCl and NAtural Deep Eutectic Solvent (NADES) medium and Ultrasound-Assisted (UA)-DLLME with beta-cyclodextrin medium were optimized. Results: The optimal DLLME conditions were found to be: 10 mg of the sample, 10% NaCl, 15% NADES or 1% beta-cyclodextrin as extraction solvent-400 muL of ethyl acetate as dispersive solvent-300 muL of ethanol, vortex time-30 s, extraction time-1 min, centrifugation at 12000x g for 5 min. Conclusions: These results were compared with microwave-assisted extraction procedures. G. purpureum and G. verum extracts showed the highest total phenolic and flavonoid content, respectively. The most potent extract in terms of antioxidant capacity was obtained from G. purpureum, whereas the extract obtained from G. album exhibited the strongest inhibitory effect against tyrosinase.
Emodin induced necroptosis in the glioma cell line U251 via the TNF-alpha/RIP1/RIP3 pathway.[Pubmed:30924024]
Invest New Drugs. 2019 Mar 28. pii: 10.1007/s10637-019-00764-w.
Emodin, an Anthraquinone compound extracted from rhubarb and other traditional Chinese medicines, has been proven to have a wide range of pharmacological effects, such as anti-inflammatory, antiviral, and antitumor activities. Previous studies have confirmed that emodin has inhibitory effects on various solid tumors, such as osteosarcoma, liver cancer, prostate cancer and glioma. This study aimed to investigate the effects and mechanisms of emodin-induced necroptosis in the glioma cell line U251 by targeting the TNF-alpha/RIP1/RIP3 signaling pathway. We found that emodin could significantly inhibit U251 cell proliferation, and the viability of U251 cells treated with emodin was reduced in a dose- and time-dependent manner. Flow cytometry assays and Hoechst-PI staining assays showed that emodin induced apoptosis and necroptosis. Real-time PCR and western blot analysis showed that emodin upregulated the levels of TNF-alpha, RIP1, RIP3 and MLKL. Furthermore, the RIP1 inhibitor Nec-1 and the RIP3 inhibitor GSK872 attenuated the killing effect of emodin on U251 cells. In addition, emodin could increase the levels of TNF-alpha, RIP1, RIP3 and MLKL in vivo. The results demonstrate that emodin could induce necroptosis in glioma possibly through the activation of the TNF-alpha/RIP1/RIP3 axis. These studies provide novel insight into the induction of necroptosis by emodin and indicate that emodin might be a potential candidate for treating glioma through the necroptosis pathway.
The role of electron shuttle enhances Fe(III)-mediated reduction of Cr(VI) by Shewanella oneidensis MR-1.[Pubmed:30923928]
World J Microbiol Biotechnol. 2019 Mar 28;35(4):64.
Chromate is one of the hazardous toxic pollutants. Reduction of Cr(VI) to Cr(III) has shown to reduce the toxicity of chromate. This work examined the reduction of Cr(VI) using an anaerobic batch cultures of Shewanella oneidensis MR-1 containing Fe(III). To do so, 10 mg/L Cr(VI) was reduced to Cr(III) within 3 days along with the oxidization of Fe(II) to Fe(III). The removal rate of Cr(VI) increased with increasing the concentration of Fe(III). In the absence of Cr(VI), the Fe(II) concentration of the batch culture increased with the growth of S. oneidensis MR-1. These data showed that S. oneidensis MR-1 could reduce Fe(III) into Fe(II), resulting in reduction of Cr(VI) to Cr(III). During this process, the Anthraquinone-2,6-disulfonate (AQDS) acted as an electron shuttle. Microscopic analysis showed that Cr(VI) had toxic effects on S. oneidensis MR-1 due to the appearance of Cr species on the bacterial surface. Cr2O3 or Cr(OH)3 precipitates formed during Cr(VI) reduction was identified using X-ray photoelectron spectroscopy. The AQDS as an electron shuttle enhanced the Cr(VI) reduction by S. oneidensis MR-1. Microbial reduction of Cr(VI) can be a useful technique for Cr detoxification.
Improvement of the electrochemical and singlet fission properties of anthraquinones by modification of the diradical character.[Pubmed:30919841]
Phys Chem Chem Phys. 2019 Apr 21;21(15):7941-7952.
In this work, theoretical methods of quantum chemistry are employed to estimate the effects that the structural modification of 1,5- and 9,10-Anthraquinone molecules might produce in their electronic structure, in the pursuit of a common strategy to improve the electrochemical and singlet fission features of conjugated quinones. The proposed modifications are the following: (i) substitution of the carbonyl oxygen atom, (ii) insertion of heteroatoms in the carbon backbone, and (iii) introduction of electron-withdrawing and electron-donating substituents in different positions of the rings. This work shows how specific modifications of the electronic structure can be used to tune the electrochemistry and photophysics of the quinones, improving their suitability to be singlet fission sensitizers and cathode materials. As it was previously known, intermediate diradical characters favor the accomplishment of the singlet fission process. This can be achieved for 1,5-Anthraquinone by introduction of B and Si atoms and electron-donating groups in low spin density positions of the carbon backbone or N atoms in high spin density positions. The introduction of heteroatoms or substituents in 9,10-Anthraquinone hardly facilitates the singlet fission process. Regarding the electrochemistry, it is observed that the reduction potentials greatly depend on the nature of the modification rather than on the diradical character.
Bis(2-pyridylmethyl)amine-functionalized alizarin: an efficient and simple colorimetric sensor for fluoride and a fluorescence turn-on sensor for Al(3+) in an organic solution.[Pubmed:30916697]
Dalton Trans. 2019 Apr 9;48(15):5035-5047.
A complexone analog chemosensor, H2L, bearing chelating bis(2-pyridylmethyl) amine and alizarin groups was synthesized via the Mannich reaction. H2L chromically responds to OH-, F-, CH3COO-, and H2PO4- in DMF, CH3CN, and acetone, but not in CH3OH or H2O. The addition of F- ions to H2L selectively induces a significant and visible color change in acetonitrile and shifts both methylene proton signals upfield. H2L also exhibits visible responses to Mg2+, Sr2+, Ba2+, Tb3+, Cu2+, Co2+, Ni2+, Zn2+, Mn2+, Cd2+, and Fe3+ in solution. AlCl3 can form an Al : L = 2 : 3 complex that not only changes the color of the DMF solution, but also significantly increases its fluorescence intensity. The limit of fluorescence turn-on detection for AlCl3 in DMF is 2.7 x 10-8 M, which is an order higher than those of other Anthraquinone sensors reported in the literature. NMR spectroscopy shows that hydroxyl is not deprotonated upon interacting with Al3+, but will be partially deprotonated in the presence of Zn2+. Contrary to the complexone, the H2L-Ce(iii) complex does not react chromically to F-. However, the H2L-NiCl2 complex responds chromically to F-, with higher sensitivity (LOD = 1.3 x 10-6 M F- in acetonitrile) than free H2L. The spectral changes in the presence of F- are similar to that of OH-; however, the spectrum shifts slightly to a longer wavelength and is more sensitive to both H2L and the H2L-NiCl2 complex. Moreover, 4% or less H2O in the solvent essentially has no influence on the F- sensitivity; however, high water content significantly decreases the F- sensitivity. The spectral changes of the Zn2+, Cu2+, Fe3+, Ce3+, and Ni2+ complexes in the presence of different NaOH concentrations were also investigated.


