Vitamin K1CAS# 84-80-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 84-80-0 | SDF | Download SDF |
| PubChem ID | 5280483 | Appearance | Yellow oil |
| Formula | C31H46O2 | M.Wt | 450.7 |
| Type of Compound | Quinones | Storage | Desiccate at -20°C |
| Synonyms | Phylloquinone; Phytomenadione | ||
| Solubility | Ethanol : ≥ 50 mg/mL (110.94 mM) DMSO : 5.6 mg/mL (12.43 mM; Need ultrasonic and warming) *"≥" means soluble, but saturation unknown. | ||
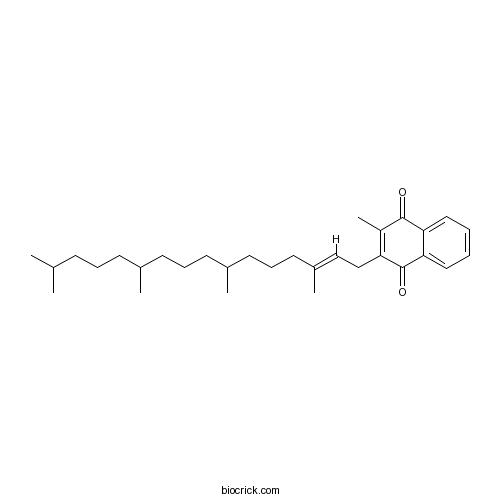
| Chemical Name | 2-methyl-3-[(E)-3,7,11,15-tetramethylhexadec-2-enyl]naphthalene-1,4-dione | ||
| SMILES | CC1=C(C(=O)C2=CC=CC=C2C1=O)CC=C(C)CCCC(C)CCCC(C)CCCC(C)C | ||
| Standard InChIKey | MBWXNTAXLNYFJB-LKUDQCMESA-N | ||
| Standard InChI | InChI=1S/C31H46O2/c1-22(2)12-9-13-23(3)14-10-15-24(4)16-11-17-25(5)20-21-27-26(6)30(32)28-18-7-8-19-29(28)31(27)33/h7-8,18-20,22-24H,9-17,21H2,1-6H3/b25-20+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Vitamin K1, a fat-soluble, naturally occurring vitamin required for blood coagulation and bone and vascular metabolism. Vitamin K1 has anticoagulation activity, it may attenuate liver fibrosis by inhibiting hepatic stellate cell activation in bile duct-ligated rats; it inhibits diabetic-cataract by modulating lens Ca2+ homeostasis and its hypoglycemic effect through its direct action on the pancreas.Vitamin K1 may substantially contribute to reducing postmenopausal bone loss at the site of the femoral neck. |
| Targets | Calcium Channel | ATPase |
| In vitro | Intestinal scavenger receptors are involved in vitamin K1 absorption.[Pubmed: 25228690]J Biol Chem. 2014 Oct 31;289(44):30743-52.Vitamin K1 (phylloquinone) intestinal absorption is thought to be mediated by a carrier protein that still remains to be identified. |
| In vivo | Inhibition of diabetic-cataract by vitamin K1 involves modulation of hyperglycemia-induced alterations to lens calcium homeostasis.[Pubmed: 25257692]Exp Eye Res. 2014 Nov;128:73-82.This study investigated the potential of Vitamin K1 against streptozotocin-induced diabetic cataract in Wistar rats.
Vitamin K1 attenuates bile duct ligation-induced liver fibrosis in rats.[Pubmed: 24742111]Scand J Gastroenterol. 2014 Jun;49(6):715-21.Vitamin K1 is used as a liver protection drug for cholestasis-induced liver fibrosis in China, but the mechanism of Vitamin K1's action in liver fibrosis is unclear.
Vitamin K1 to slow vascular calcification in haemodialysis patients (VitaVasK trial): a rationale and study protocol.[Pubmed: 24285427]Nephrol Dial Transplant. 2014 Sep;29(9):1633-8.Patients on haemodialysis (HD) exhibit increased cardiovascular mortality associated with accelerated vascular calcification (VC). VC is influenced by inhibitors such as matrix Gla protein (MGP), a protein activated in the presence of vitamin K. HD patients exhibit marked vitamin K deficiency, and supplementation with vitamin K reduces inactive MGP levels in these patients. The VitaVasK trial analyses whether Vitamin K1 supplementation affects the progression of coronary and aortic calcification in HD patients.
Vitamin K1 supplementation retards bone loss in postmenopausal women between 50 and 60 years of age.[Pubmed: 14506950]Calcif Tissue Int. 2003 Jul;73(1):21-6.Although several observational studies have demonstrated an association between vitamin K status and bone mineral density (BMD) in postmenopausal women, no placebo-controlled intervention trials of the effect of Vitamin K1 supplementation on bone loss have been reported thus far.
Efficacy and safety of intravenous phytonadione (vitamin K1) in patients on long-term oral anticoagulant therapy.[Pubmed: 11243272 ]Mayo Clin Proc. 2001 Mar;76(3):260-6.To determine the safety and efficacy of intravenously administered phytonadione (Vitamin K1) in patients on routine oral warfarin anticoagulation.
|
| Animal Research | The severe adverse reaction to vitamin k1 injection is anaphylactoid reaction but not anaphylaxis.[Pubmed: 24594861]PLoS One. 2014 Mar 4;9(3):e90199.The severe adverse reaction to Vitamin K1 injection is always remarkable and is thought to result from anaphylaxis. Paradoxically, however, some patients administered Vitamin K1 injection for the first time have adverse reactions.
|

Vitamin K1 Dilution Calculator

Vitamin K1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2188 mL | 11.0939 mL | 22.1877 mL | 44.3754 mL | 55.4693 mL |
| 5 mM | 0.4438 mL | 2.2188 mL | 4.4375 mL | 8.8751 mL | 11.0939 mL |
| 10 mM | 0.2219 mL | 1.1094 mL | 2.2188 mL | 4.4375 mL | 5.5469 mL |
| 50 mM | 0.0444 mL | 0.2219 mL | 0.4438 mL | 0.8875 mL | 1.1094 mL |
| 100 mM | 0.0222 mL | 0.1109 mL | 0.2219 mL | 0.4438 mL | 0.5547 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Vitamin K1 a fat-soluble, naturally occurring vitamin required for blood coagulation and bone and vascular metabolism.
In Vitro:Phylloquinone (Vitamin K1) is a prenylated naphthoquinone that is synthesized exclusively by plants, green algae, and some species of cyanobacteria, where it serves as a vital electron carrier in photosystem I and as an electron acceptor for the formation of protein disulfide bonds. In humans and other vertebrates, phylloquinone plays the role of a vitamin (vitamin K1) that is required for blood coagulation and bone and vascular metabolism. Phylloquinone from green leafy vegetables and vegetable oil represents the major dietary source of vitamin K for humans[1]. Vitamin K1 treatment causes a significant antiproliferative effect and induces apoptosis in Caco-2, HT-29, and SW480 cell lines, with the involvement of the MAPK pathway. A concomitant and significant decrease in the polyamine biosynthesis occurr[2].
In Vivo:Subjects who increase their dietary intake of vitamin K during the follow-up had a 51% reduced risk of incident diabetes compared with subjects who decrease or does not change the amount of phylloquinone intake[3]. Vitamin K supplementation reverses the high fat diet induced bone deterioration by modulating osteoblast and osteoclast activities and prevent bone loss in a high-fat diet-induced obese mice[4]. Application of vitamin K1 to the skin has been used for suppression of pigmentation and resolution of bruising. The effects produced by the topical vitamin K1 shows significant healing when compared with control group in parameters such as wound contraction, epithelialization period, hydroxyproline content and tensile strength[5].
References:
[1]. Basset GJ, et al. Phylloquinone (Vitamin K1): Occurrence, Biosynthesis and Functions. Mini Rev Med Chem. 2016 Jun 22.
[2]. Orlando A, et al. Vitamin K1 exerts antiproliferative effects and induces apoptosis in three differently graded human colon cancer cell lines. Biomed Res Int. 2015;2015:296721.
[3]. Ibarrola-Jurado N, et al. Dietary phylloquinone intake and risk of type 2 diabetes in elderly subjects at high risk of cardiovascular disease. Am J Clin Nutr. 2012 Nov;96(5):1113-8.
[4]. Kim M, et al. Vitamin K1 (phylloquinone) and K2 (menaquinone-4) supplementation improves bone formation in a high-fat diet-induced obese mice. J Clin Biochem Nutr. 2013 Sep;53(2):108-13.
[5]. Hemmati AA, et al. Topical vitamin K1 promotes repair of full thickness wound in rat. Indian J Pharmacol. 2014 Jul-Aug;46(4):409-12.
- Lapachol
Catalog No.:BCN4391
CAS No.:84-79-7
- Dibutyl Phthalate
Catalog No.:BCC8411
CAS No.:84-74-2
- Diisobutyl phthalate
Catalog No.:BCN7148
CAS No.:84-69-5
- Anthraquinone
Catalog No.:BCC8832
CAS No.:84-65-1
- Anthraflavic acid
Catalog No.:BCC8831
CAS No.:84-60-6
- Tectoquinone
Catalog No.:BCN3481
CAS No.:84-54-8
- Stylopine
Catalog No.:BCN3715
CAS No.:84-39-9
- Syrosingopine
Catalog No.:BCN5365
CAS No.:84-36-6
- Ophiohayatone C
Catalog No.:BCN3608
CAS No.:84-33-3
- Rutaecarpine
Catalog No.:BCN4385
CAS No.:84-26-4
- Hexestrol
Catalog No.:BCC4484
CAS No.:84-16-2
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
- Xanthoxyletin
Catalog No.:BCN6579
CAS No.:84-99-1
- Fmoc-N-Me-Ala-OH
Catalog No.:BCC3210
CAS No.:84000-07-7
- Fmoc-N-Me-Val-OH
Catalog No.:BCC3358
CAS No.:84000-11-3
- Helioxanthin 8-1
Catalog No.:BCC5415
CAS No.:840529-13-7
- Lamotrigine
Catalog No.:BCC5051
CAS No.:84057-84-1
- Roquinimex
Catalog No.:BCC5355
CAS No.:84088-42-6
- 1-Benzhydrylpiperazine
Catalog No.:BCC8453
CAS No.:841-77-0
- Wilforlide A
Catalog No.:BCN4383
CAS No.:84104-71-2
- Wilforlide A acetate
Catalog No.:BCN4384
CAS No.:84104-80-3
- Triptotriterpenic acid A
Catalog No.:BCN6780
CAS No.:84108-17-8
- R406 (free base)
Catalog No.:BCC2553
CAS No.:841290-80-0
- R406
Catalog No.:BCC3876
CAS No.:841290-81-1
Intestinal scavenger receptors are involved in vitamin K1 absorption.[Pubmed:25228690]
J Biol Chem. 2014 Oct 31;289(44):30743-52.
Vitamin K1 (phylloquinone) intestinal absorption is thought to be mediated by a carrier protein that still remains to be identified. Apical transport of Vitamin K1 was examined using Caco-2 TC-7 cell monolayers as a model of human intestinal epithelium and in transfected HEK cells. Phylloquinone uptake was then measured ex vivo using mouse intestinal explants. Finally, Vitamin K1 absorption was compared between wild-type mice and mice overexpressing scavenger receptor class B type I (SR-BI) in the intestine and mice deficient in cluster determinant 36 (CD36). Phylloquinone uptake by Caco-2 cells was saturable and was significantly impaired by co-incubation with alpha-tocopherol (and vice versa). Anti-human SR-BI antibodies and BLT1 (a chemical inhibitor of lipid transport via SR-BI) blocked up to 85% of Vitamin K1 uptake. BLT1 also decreased phylloquinone apical efflux by approximately 80%. Transfection of HEK cells with SR-BI and CD36 significantly enhanced Vitamin K1 uptake, which was subsequently decreased by the addition of BLT1 or sulfo-N-succinimidyl oleate (CD36 inhibitor), respectively. Similar results were obtained in mouse intestinal explants. In vivo, the phylloquinone postprandial response was significantly higher, and the proximal intestine mucosa phylloquinone content 4 h after gavage was increased in mice overexpressing SR-BI compared with controls. Phylloquinone postprandial response was also significantly increased in CD36-deficient mice compared with wild-type mice, but their Vitamin K1 intestinal content remained unchanged. Overall, the present data demonstrate for the first time that intestinal scavenger receptors participate in the absorption of dietary phylloquinone.
Vitamin K1 to slow vascular calcification in haemodialysis patients (VitaVasK trial): a rationale and study protocol.[Pubmed:24285427]
Nephrol Dial Transplant. 2014 Sep;29(9):1633-8.
BACKGROUND: Patients on haemodialysis (HD) exhibit increased cardiovascular mortality associated with accelerated vascular calcification (VC). VC is influenced by inhibitors such as matrix Gla protein (MGP), a protein activated in the presence of vitamin K. HD patients exhibit marked vitamin K deficiency, and supplementation with vitamin K reduces inactive MGP levels in these patients. The VitaVasK trial analyses whether Vitamin K1 supplementation affects the progression of coronary and aortic calcification in HD patients. METHODS: VitaVasK is a prospective, randomized, parallel group, multicentre trial (EudraCT No.: 2010-021264-14) that will include 348 HD patients in an open-label, two-arm design. After baseline multi-slice computed tomography (MSCT) of the heart and thoracic aorta, patients with a coronary calcification volume score of at least 100 will be randomized to continue on standard care or to receive additional supplementation with 5 mg Vitamin K1 orally thrice weekly. Treatment duration will be 18 months, and MSCT scans will be repeated after 12 and 18 months. Primary end points are the progression of thoracic aortic and coronary artery calcification (calculated as absolute changes in the volume scores at the 18-month MSCT versus the baseline MSCT). Secondary end points comprise changes in Agatston score, mitral and aortic valve calcification as well as major adverse cardiovascular events (MACE) and all-cause mortality. VitaVask also aims to record MACE and all-cause mortality in the follow-up period at 3 and 5 years after treatment initiation. This trial may lead to the identification of an inexpensive and safe treatment or prophylaxis of VC in HD patients.
Vitamin K1 attenuates bile duct ligation-induced liver fibrosis in rats.[Pubmed:24742111]
Scand J Gastroenterol. 2014 Jun;49(6):715-21.
Vitamin K1 is used as a liver protection drug for cholestasis-induced liver fibrosis in China, but the mechanism of Vitamin K1's action in liver fibrosis is unclear. In this study, a model of liver fibrosis was achieved via bile duct ligation in rats. The rats were then injected with Vitamin K1, and the levels of serum aspartate aminotransferase, alanine transaminase, total bilirubin and the fibrotic grade score, collagen content, the expressions of alpha-smooth muscle actin (SMA) and cytokeratin 19 (CK19) were measured on day 28 after ligation. The levels of the biochemical parameters, fibrotic score and collagen content were significantly reduced by treatment with Vitamin K1 in bile duct-ligated rats. In addition, alpha-SMA and CK19 expression was significantly reduced by Vitamin K1 treatment in bile duct-ligated rats. These results suggested that Vitamin K1 may attenuate liver fibrosis by inhibiting hepatic stellate cell activation in bile duct-ligated rats.
Inhibition of diabetic-cataract by vitamin K1 involves modulation of hyperglycemia-induced alterations to lens calcium homeostasis.[Pubmed:25257692]
Exp Eye Res. 2014 Nov;128:73-82.
This study investigated the potential of Vitamin K1 against streptozotocin-induced diabetic cataract in Wistar rats. A single, intraperitoneal injection of streptozotocin (STZ) (35 mg/kg) resulted in hyperglycemia, accumulation of sorbitol and formation of advanced glycation end product (AGE) in eye lens. Hyperglycemia in lens also resulted in superoxide anion and hydroxyl radical generation and less reduced glutathione suggesting oxidative stress in lens. Hyperglycemia also resulted in increase in lens Ca2+ and significant inhibition of lens Ca2+ ATPase activity. These changes were associated with cataract formation in diabetic animals. By contrast treatment of diabetic rats with Vitamin K1 (5 mg/kg, sc, twice a week) resulted in animals with partially elevated blood glucose and with transparent lenses having normal levels of sorbitol, AGE, Ca2+ ATPase, Ca2+, and oxidative stress. Vitamin K 1 may function to protect against cataract formation in the STZ induced diabetic rat by affecting the homeostasis of blood glucose and minimizing subsequent oxidative and osmotic stress. Thus, these results show that Vitamin K1 inhibits diabetic-cataract by modulating lens Ca2+ homeostasis and its hypoglycemic effect through its direct action on the pancreas.
Efficacy and safety of intravenous phytonadione (vitamin K1) in patients on long-term oral anticoagulant therapy.[Pubmed:11243272]
Mayo Clin Proc. 2001 Mar;76(3):260-6.
OBJECTIVES: To determine the safety and efficacy of intravenously administered phytonadione (Vitamin K1) in patients on routine oral warfarin anticoagulation. PATIENTS AND METHODS: This retrospective cohort study comprised adults who were taking warfarin, were not bleeding, and received intravenous phytonadione anticoagulation therapy before a diagnostic or therapeutic procedure between September 1, 1994, and March 31, 1996. The main outcome measures were adverse reactions to intravenously administered phytonadione, prothrombin-international normalized ratio time values, the incidence of bleeding and thrombosis after the procedure, and the time between the procedure and return to anticoagulation after resumption of warfarin treatment. RESULTS: Two (1.9%) of the 105 patients studied had suspected adverse reactions to intravenous phytonadione (dyspnea and chest tightness during infusion in both). For the 82 patients who underwent a procedure, the median time from phytonadione to procedure onset was 27 hours (range, 0.7-147 hours), which was significantly less for patients receiving an initial phytonadione dose of more than 1 mg (P=.009). None had thromboembolism after surgery, although 2 (2.4%) of the 82 patients had procedure-associated major bleeding. For the 60 patients resuming warfarin therapy after a procedure, the median time to return to therapeutic anticoagulation was 4.1 days (range, 0.8-31.7 days) and was unaffected by the phytonadione dosage. CONCLUSIONS: Intravenous phytonadione appears to be safe and is effective for semiurgent correction of long-term oral anticoagulation therapy before surgery. In small doses, it does not prolong the patient's time to return to therapeutic anticoagulation.
Vitamin K1 supplementation retards bone loss in postmenopausal women between 50 and 60 years of age.[Pubmed:14506950]
Calcif Tissue Int. 2003 Jul;73(1):21-6.
Although several observational studies have demonstrated an association between vitamin K status and bone mineral density (BMD) in postmenopausal women, no placebo-controlled intervention trials of the effect of Vitamin K1 supplementation on bone loss have been reported thus far. In the trial presented here we have investigated the potential complementary effect of Vitamin K1 (1 mg/day) and a mineral + vitamin D supplement (8 microg/day) on postmenopausal bone loss. The design of our study was a randomized, double-blind, placebo-controlled intervention study; 181 healthy postmenopausal women between 50 and 60 years old were recruited, 155 of whom completed the study. During the 3-year treatment period, participants received a daily supplement containing either placebo, or calcium, magnesium, zinc, and vitamin D (MD group), or the same formulation with additional Vitamin K1 (MDK group). The main outcome was the change in BMD of the femoral neck and lumbar spine after 3 years, as measured by DXA. The group receiving the supplement containing additional Vitamin K1 showed reduced bone loss of the femoral neck: after 3 years the difference between the MDK and the placebo group was 1.7% (95% Cl: 0.35-3.44) and that between the MDK and MD group was 1.3% (95% Cl: 0.10-3.41). No significant differences were observed among the three groups with respect to change of BMD at the site of the lumbar spine. If co-administered with minerals and vitamin D, Vitamin K1 may substantially contribute to reducing postmenopausal bone loss at the site of the femoral neck.
The severe adverse reaction to vitamin k1 injection is anaphylactoid reaction but not anaphylaxis.[Pubmed:24594861]
PLoS One. 2014 Mar 4;9(3):e90199.
The severe adverse reaction to Vitamin K1 injection is always remarkable and is thought to result from anaphylaxis. Paradoxically, however, some patients administered Vitamin K1 injection for the first time have adverse reactions. Using beagle dogs, the present study tested the hypothesis that the response to Vitamin K1 is an anaphylactoid reaction. The results showed that serious anaphylaxis-like symptoms appeared in beagle dogs after the administration of Vitamin K1 injection for the first time. The plasma histamine concentration increased, and blood pressure decreased sharply. After sensitization, dogs were challenged with Vitamin K1 injection and displayed the same degree of symptoms as prior to sensitization. However, when the Vitamin K1 injection-sensitized dogs were challenged with a Vitamin K1-fat emulsion without solubilizers such asTween-80, the abnormal reactions did not occur. Furthermore, there was no significant change in the plasma immunoglobulin E concentration after Vitamin K1 challenge. Following treatment with Vitamin K1 injection, the release of histamine and beta-hexosaminidase by rat basophilic leukemia-2H3 cells as well as the rate of apoptosis increased. The Tween-80 group displayed results similar to those observed following Vitamin K1 injection in vivo. However, the dogs in the Vitamin K1-fat emulsion group did not display any abnormal behavior or significant change in plasma histamine. Additionally, degranulation and apoptosis did not occur in rat basophilic leukemia-2H3 cells. Our results indicate that the adverse reaction induced by Vitamin K1 injection is an anaphylactoid reaction, not anaphylaxis. Vitamin K1 injection induces the release of inflammatory factors via a non-IgE-mediated immune pathway, for which the trigger may be the solubilizer.


