Baccatin IIICAS# 27548-93-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 27548-93-2 | SDF | Download SDF |
| PubChem ID | 65366 | Appearance | White powder |
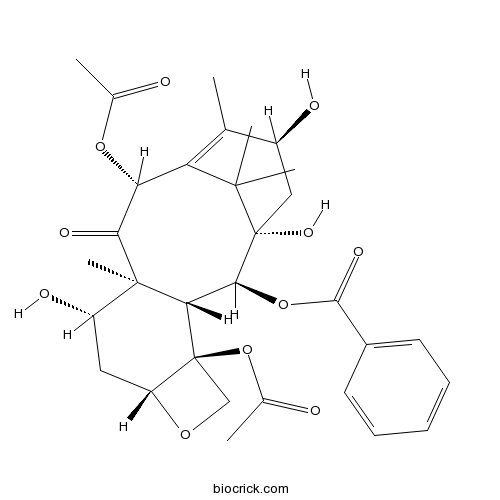
| Formula | C31H38O11 | M.Wt | 586.6 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | DMSO : 125 mg/mL (213.08 mM; Need ultrasonic) | ||
| SMILES | CC1=C2C(C(=O)C3(C(CC4C(C3C(C(C2(C)C)(CC1O)O)OC(=O)C5=CC=CC=C5)(CO4)OC(=O)C)O)C)OC(=O)C | ||
| Standard InChIKey | OVMSOCFBDVBLFW-VHLOTGQHSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Baccatin III, which is the precursor for the semisynthesis of paclitaxel,exerts anti-tumor immunomodulatory activity in very low doses (0.05-0.5mg/kg),it reduces tumor progression by inhibiting the accumulation and suppressive function of MDSCs. Baccatin III also exerts immunomodulatory activities in vivo as well as in vitro on the MHC-restricted antigen presentation. |
| Targets | Caspase | Bcl-2/Bax |
| In vitro | Baccatin III, a precursor for the semisynthesis of paclitaxel, inhibits the accumulation and suppressive activity of myeloid-derived suppressor cells in tumor-bearing mice.[Pubmed: 24957690]Int Immunopharmacol. 2014 Aug;21(2):487-93.Myeloid-derived suppressor cells (MDSCs) mediate tumor-associated immune suppression in both cancer patients and tumor-bearing animals. Reduction or elimination of MDSCs reduces the rate of tumor progression and improves cancer therapies that employ mechanisms of immunity. |
| In vivo | Baccatin III, a synthetic precursor of taxol, enhances MHC-restricted antigen presentation in dendritic cells.[Pubmed: 21354357]Int Immunopharmacol. 2011 Aug;11(8):985-91.Baccatin III, a precursor for the semisynthesis of taxol, is widely considered to be an inactive derivative of taxol. Here we show that Baccatin III efficiently enhances MHC-restricted antigen presentation in dendritic cells. |
| Cell Research | Inhibition of cancer cell proliferation and apoptosis-inducing activity of fungal taxol and its precursor baccatin III purified from endophytic Fusarium solani.[Pubmed: 24152585 ]Cancer Cell Int. 2013 Oct 23;13(1):105.Taxol (generic name paclitaxel), a plant-derived antineoplastic agent, used widely against breast, ovarian and lung cancer, was originally isolated from the bark of the Pacific yew, Taxus brevifolia. The limited supply of the drug has prompted efforts to find alternative sources, such as chemical synthesis, tissue and cell cultures of the Taxus species both of which are expensive and yield low levels. Fermentation processes with microorganisms would be the methods of choice to lower the costs and increase yields. Previously we have reported that F. solani isolated from T. celebica produced taxol and its precursor Baccatin III in liquid grown cultures J Biosci 33:259-67, 2008. This study was performed to evaluate the inhibition of proliferation and induction of apoptosis of cancer cell lines by the fungal taxol and fungal Baccatin III of F. solani isolated from T. celebica.
|
| Structure Identification | J Sci Food Agric. 2014 Sep;94(12):2376-83.Bioproduction of baccatin III, an advanced precursor of paclitaxol, with transgenic Flammulina velutipes expressing the 10-deacetylbaccatin III-10-O-acetyl transferase gene.[Pubmed: 24403190]10-DeacetylBaccatin III (10-DAB) and Baccatin III are intermediates in the biosynthesis of Taxol (an anti-cancer drug) and useful precursors for semi-synthesis of the drug. |

Baccatin III Dilution Calculator

Baccatin III Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7047 mL | 8.5237 mL | 17.0474 mL | 34.0948 mL | 42.6185 mL |
| 5 mM | 0.3409 mL | 1.7047 mL | 3.4095 mL | 6.819 mL | 8.5237 mL |
| 10 mM | 0.1705 mL | 0.8524 mL | 1.7047 mL | 3.4095 mL | 4.2618 mL |
| 50 mM | 0.0341 mL | 0.1705 mL | 0.3409 mL | 0.6819 mL | 0.8524 mL |
| 100 mM | 0.017 mL | 0.0852 mL | 0.1705 mL | 0.3409 mL | 0.4262 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- MS 245 oxalate
Catalog No.:BCC6127
CAS No.:275363-58-1
- H-Gly-OtBu.HCl
Catalog No.:BCC2952
CAS No.:27532-96-3
- Feretoside
Catalog No.:BCN5164
CAS No.:27530-67-2
- Gedunin
Catalog No.:BCC7676
CAS No.:2753-30-2
- H-Cha-OH
Catalog No.:BCC2663
CAS No.:27527-05-5
- Gambogic acid
Catalog No.:BCN2318
CAS No.:2752-65-0
- Mesaconitine
Catalog No.:BCN5987
CAS No.:2752-64-9
- O-Methylpallidine
Catalog No.:BCN3916
CAS No.:27510-33-4
- Physalin C
Catalog No.:BCN7918
CAS No.:27503-33-9
- Protostemonine
Catalog No.:BCN8172
CAS No.:27495-40-5
- Vildagliptin (LAF-237)
Catalog No.:BCC2112
CAS No.:274901-16-5
- 2-O-Acetyltutin
Catalog No.:BCN5163
CAS No.:2749-28-2
- MMF
Catalog No.:BCC7941
CAS No.:2756-87-8
- Communic acid
Catalog No.:BCN5166
CAS No.:2761-77-5
- Danaidal
Catalog No.:BCN1965
CAS No.:27628-46-2
- Rhodexin B
Catalog No.:BCC8246
CAS No.:2763-20-4
- Muscimol
Catalog No.:BCC6593
CAS No.:2763-96-4
- 15-Nonacosanol
Catalog No.:BCC8439
CAS No.:2764-81-0
- 5-Isoquinolinesulfonic acid
Catalog No.:BCC8749
CAS No.:27655-40-9
- Boc-Ser-OMe
Catalog No.:BCC3441
CAS No.:2766-43-0
- Cyanidin-3-O-galactoside chloride
Catalog No.:BCN3022
CAS No.:27661-36-5
- Leucoside
Catalog No.:BCN5167
CAS No.:27661-51-4
- Akt/SKG Substrate Peptide
Catalog No.:BCC5748
CAS No.:276680-69-4
- WAY 161503 hydrochloride
Catalog No.:BCC7179
CAS No.:276695-22-8
Baccatin III, a precursor for the semisynthesis of paclitaxel, inhibits the accumulation and suppressive activity of myeloid-derived suppressor cells in tumor-bearing mice.[Pubmed:24957690]
Int Immunopharmacol. 2014 Aug;21(2):487-93.
Myeloid-derived suppressor cells (MDSCs) mediate tumor-associated immune suppression in both cancer patients and tumor-bearing animals. Reduction or elimination of MDSCs reduces the rate of tumor progression and improves cancer therapies that employ mechanisms of immunity. Here we show that Baccatin III, which is the precursor for the semisynthesis of paclitaxel, exerts anti-tumor immunomodulatory activity in very low doses (0.05-0.5mg/kg), although it is regarded as an inactive derivative of paclitaxel. Oral administration of Baccatin III significantly reduced the growth of tumors induced by engrafting BALB/c mice with either 4 T1 mammary carcinoma or CT26 colon cancer cells. Baccatin III (0.5mg/kg) did not exert anti-tumor activity in athymic nude mice. Baccatin III decreased the accumulation of MDSCs in the spleens of the tumor-bearing mice. Furthermore, MDSCs isolated from Baccatin III-treated mice, compared with those isolated from vehicle-treated mice, had a significantly reduced suppressive effect on T cells treated with the anti-CD3 and anti-CD28 monoclonal antibodies. Moreover, these cells produced significantly reduced amounts of reactive oxygen species and nitric oxide. These results suggest that Baccatin III reduced tumor progression by inhibiting the accumulation and suppressive function of MDSCs.
Bioproduction of baccatin III, an advanced precursor of paclitaxol, with transgenic Flammulina velutipes expressing the 10-deacetylbaccatin III-10-O-acetyl transferase gene.[Pubmed:24403190]
J Sci Food Agric. 2014 Sep;94(12):2376-83.
BACKGROUND: 10-DeacetylBaccatin III (10-DAB) and Baccatin III are intermediates in the biosynthesis of Taxol (an anti-cancer drug) and useful precursors for semi-synthesis of the drug. In this study, a bioconversion system was established for the production of Baccatin III, an advanced precursor of paclitaxel, in the transgenic mushroom Flammulina velutipes expressing the 10-deacetylBaccatin III-10beta-O-acetyltransferase gene. The expression vector pgFvs-TcDBAT containing the 10-deacetylBaccatin III-10beta-O-acetyltransferase (DBAT) gene was constructed and transformed into the cells of F. velutipes by polyethylene glycol-mediated protoplast transformation. RESULTS: Polymerase chain reaction and Southern blotting analysis verified the successful integration of the exogenous DBAT gene into the genome of F. velutipes. Reverse transcription polymerase chain reaction and enzyme activity analyses confirmed that the DBAT gene was expressed in F. velutipes, and DBAT is able to convert substrate into Baccatin III. CONCLUSION: The DBAT gene from the plant Taxus chinensis can be functionally expressed in F. velutipes. Transgenic F. velutipes expressing the DBAT gene is able to produce the target product, Baccatin III. This is the first report about the transformation and expression of paclitaxel biosynthetic gene in the edible mushroom F. velutipes. This represents a significant step towards bio-production of paclitaxel and its advanced precursor Baccatin III in an edible fungus.
Baccatin III, a synthetic precursor of taxol, enhances MHC-restricted antigen presentation in dendritic cells.[Pubmed:21354357]
Int Immunopharmacol. 2011 Aug;11(8):985-91.
Baccatin III, a precursor for the semisynthesis of taxol, is widely considered to be an inactive derivative of taxol. Here we show that Baccatin III efficiently enhances MHC-restricted antigen presentation in dendritic cells. Baccatin III increased both class I- and class II-restricted presentation of exogenous OVA in bone marrow-derived dendritic cells (BM-DCs). Baccatin III also increased class I-restricted presentation of virus-encoded endogenous OVA in BM-DCs. Baccatin III did not affect the phagocytic activity of BM-DCs. The antigen presentation-enhancing activity of Baccatin III was examined further with nanoparticles containing OVA and Baccatin III. Inclusion of Baccatin III to nanoparticles containing OVA greatly enhanced their capacity to induce class I-restricted OVA peptide presentation in DCs both in vitro and in vivo. Accordingly, nanoparticles containing both OVA and Baccatin III were much more efficient in inducing an OVA-specific CTL response in mice compared to those containing OVA only. These results demonstrate that Baccatin III exerts immunomodulatory activities in vivo as well as in vitro on the MHC-restricted antigen presentation.
Inhibition of cancer cell proliferation and apoptosis-inducing activity of fungal taxol and its precursor baccatin III purified from endophytic Fusarium solani.[Pubmed:24152585]
Cancer Cell Int. 2013 Oct 23;13(1):105.
BACKGROUND: Taxol (generic name paclitaxel), a plant-derived antineoplastic agent, used widely against breast, ovarian and lung cancer, was originally isolated from the bark of the Pacific yew, Taxus brevifolia. The limited supply of the drug has prompted efforts to find alternative sources, such as chemical synthesis, tissue and cell cultures of the Taxus species both of which are expensive and yield low levels. Fermentation processes with microorganisms would be the methods of choice to lower the costs and increase yields. Previously we have reported that F. solani isolated from T. celebica produced taxol and its precursor Baccatin III in liquid grown cultures J Biosci 33:259-67, 2008. This study was performed to evaluate the inhibition of proliferation and induction of apoptosis of cancer cell lines by the fungal taxol and fungal Baccatin III of F. solani isolated from T. celebica. METHODS: Cell lines such as HeLa, HepG2, Jurkat, Ovcar3 and T47D were cultured individually and treated with fungal taxol, Baccatin III with or without caspase inhibitors according to experimental requirements. Their efficacy on apoptotic induction was examined. RESULTS: Both fungal taxol and Baccatin III inhibited cell proliferation of a number of cancer cell lines with IC50 ranging from 0.005 to 0.2 muM for fungal taxol and 2 to 5 muM for fungal Baccatin III. They also induced apoptosis in JR4-Jurkat cells with a possible involvement of anti-apoptotic Bcl2 and loss in mitochondrial membrane potential, and was unaffected by inhibitors of caspase-9,-2 or -3 but was prevented in presence of caspase-10 inhibitor. DNA fragmentation was also observed in cells treated with fungal taxol and Baccatin III. CONCLUSIONS: The cytotoxic activity exhibited by fungal taxol and Baccatin III involves the same mechanism, dependent on caspase-10 and membrane potential loss of mitochondria, with taxol having far greater cytotoxic potential.


