MMFActivator of Nrf2 pathway; GPR109A agonist CAS# 2756-87-8 |
- Mc-MMAD
Catalog No.:BCC1735
CAS No.:1401963-15-2
- Nocodazole
Catalog No.:BCC3826
CAS No.:31430-18-9
- Colchicine
Catalog No.:BCN6271
CAS No.:64-86-8
- Mc-MMAE
Catalog No.:BCC5201
CAS No.:863971-24-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2756-87-8 | SDF | Download SDF |
| PubChem ID | 5354456 | Appearance | Powder |
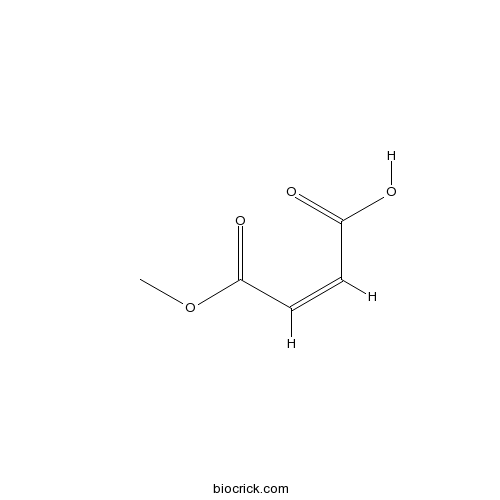
| Formula | C5H6O4 | M.Wt | 130.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | mono-Methyl fumarate | ||
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
| Chemical Name | (Z)-4-methoxy-4-oxobut-2-enoic acid | ||
| SMILES | COC(=O)C=CC(=O)O | ||
| Standard InChIKey | NKHAVTQWNUWKEO-IHWYPQMZSA-N | ||
| Standard InChI | InChI=1S/C5H6O4/c1-9-5(8)3-2-4(6)7/h2-3H,1H3,(H,6,7)/b3-2- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Nuclear factor (erythroid-derived-2)-like 2 (Nrf2) pathway activator. Also exhibits agonist activity at GPR109A. Primary metabolite of DMF. |

MMF Dilution Calculator

MMF Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.6864 mL | 38.432 mL | 76.864 mL | 153.7279 mL | 192.1599 mL |
| 5 mM | 1.5373 mL | 7.6864 mL | 15.3728 mL | 30.7456 mL | 38.432 mL |
| 10 mM | 0.7686 mL | 3.8432 mL | 7.6864 mL | 15.3728 mL | 19.216 mL |
| 50 mM | 0.1537 mL | 0.7686 mL | 1.5373 mL | 3.0746 mL | 3.8432 mL |
| 100 mM | 0.0769 mL | 0.3843 mL | 0.7686 mL | 1.5373 mL | 1.9216 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Baccatin III
Catalog No.:BCN5165
CAS No.:27548-93-2
- MS 245 oxalate
Catalog No.:BCC6127
CAS No.:275363-58-1
- H-Gly-OtBu.HCl
Catalog No.:BCC2952
CAS No.:27532-96-3
- Feretoside
Catalog No.:BCN5164
CAS No.:27530-67-2
- Gedunin
Catalog No.:BCC7676
CAS No.:2753-30-2
- H-Cha-OH
Catalog No.:BCC2663
CAS No.:27527-05-5
- Gambogic acid
Catalog No.:BCN2318
CAS No.:2752-65-0
- Mesaconitine
Catalog No.:BCN5987
CAS No.:2752-64-9
- O-Methylpallidine
Catalog No.:BCN3916
CAS No.:27510-33-4
- Physalin C
Catalog No.:BCN7918
CAS No.:27503-33-9
- Protostemonine
Catalog No.:BCN8172
CAS No.:27495-40-5
- Vildagliptin (LAF-237)
Catalog No.:BCC2112
CAS No.:274901-16-5
- Communic acid
Catalog No.:BCN5166
CAS No.:2761-77-5
- Danaidal
Catalog No.:BCN1965
CAS No.:27628-46-2
- Rhodexin B
Catalog No.:BCC8246
CAS No.:2763-20-4
- Muscimol
Catalog No.:BCC6593
CAS No.:2763-96-4
- 15-Nonacosanol
Catalog No.:BCC8439
CAS No.:2764-81-0
- 5-Isoquinolinesulfonic acid
Catalog No.:BCC8749
CAS No.:27655-40-9
- Boc-Ser-OMe
Catalog No.:BCC3441
CAS No.:2766-43-0
- Cyanidin-3-O-galactoside chloride
Catalog No.:BCN3022
CAS No.:27661-36-5
- Leucoside
Catalog No.:BCN5167
CAS No.:27661-51-4
- Akt/SKG Substrate Peptide
Catalog No.:BCC5748
CAS No.:276680-69-4
- WAY 161503 hydrochloride
Catalog No.:BCC7179
CAS No.:276695-22-8
- Cryptomoscatone D2
Catalog No.:BCN7203
CAS No.:276856-55-4
Tac-MMF Versus CsA-MMF/CsA-AZA-Based Regimens in Development of De Novo Complement-Binding Anti-HLA Antibodies After Kidney Transplantation.[Pubmed:28340811]
Transplant Proc. 2017 Apr;49(3):454-459.
BACKGROUND: Immunosuppressive regimens with tacrolimus or cyclosporine A (CsA) were compared for graft-related outcomes in conjunction with complement-binding de novo donor-specific antibodies (DSAs). METHODS: Non-sensitized adult patients without rejection episodes within 3 months after transplantation were screened for the presence of de novo DSAs and C1q binding. Clinical and biopsy data were retrospectively obtained. RESULTS: The analysis included 118 patients (68 tacrolimus, 50 CsA), with mean age and follow-up of 36.1 +/- 11.4 and 7.2 +/- 4.8 years, respectively. As compared with tacrolimus, the CsA group had higher rates of both class II DSAs and C1q-binding DSAs (20% vs 4.4%, P = .008, and 18% vs 0%, P = .003, respectively). Rates of chronic antibody-mediated rejection (cAMR), proteinuria >500 mg/g, and levels of creatinine both at last visits were also higher in the CsA group (20% vs 0%, P = .002, 30% vs 5.9%, P = .005, 1.67 +/- 1.31 vs 1.18 +/- 0.45 mg/dL, P = .019, respectively).Class II DSAs and C1q-binding class II DSAs were significantly correlated with the clinical outcomes (creatinine levels, proteinuria, and cAMR). CONCLUSIONS: Compared with tacrolimus, CsA appears to pose a higher risk for the development of de novo anti-HLA antibodies with C1q-binding properties and, consequently, adverse graft-related outcomes.
In-vitro influence of mycophenolate mofetil (MMF) and Ciclosporin A (CsA) on cytokine induced killer (CIK) cell immunotherapy.[Pubmed:27620209]
J Transl Med. 2016 Sep 13;14:264.
BACKGROUND: Cytokine-induced-killer (CIK) cells are a promising immunotherapeutic approach for impending relapse following hematopoietic stem cell transplantation (HSCT). However, there is a high risk for treatment failure associated with severe graft versus host disease (GvHD) necessitating pharmaceutical intervention post-transplant. Whether immunosuppression with mycophenolate mofetil (MMF) or Ciclosporin A (CsA) influences the cytotoxic effect of CIK cell immunotherapy is still an open issue. METHODS: CIK cells were generated from PBMC as previously described followed by co-incubation with mycophenolic acid (MPA) or CsA. Proliferation, cytotoxicity and receptor expression were investigated following short- (24 h), intermediate- (3 days) and long-term (7 days) MPA incubation with the intention to simulate the in vivo situation when CIK cells were given to a patient with relevant MPA/CsA plasma levels. RESULTS: Short-term MPA treatment led to unchanged proliferation capacity and barely had any effect on viability and cytotoxic capability in vitro. The composition of CIK cells with respect to T-, NK-like T- and NK cells remained stable. Intermediate MPA treatment lacked effects on NKG2D, FasL and TRAIL receptor expression, while an influence on proliferation and viability was detectable. Furthermore, long-term treatment significantly impaired proliferation, restricted viability and drastically reduced migration-relevant receptors accompanied by an alteration in the CD4/CD8 ratio. CD3(+)CD56(+) cells upregulated receptors relevant for CIK cell killing and migration, whereas T cells showed the most interference through significant reductions in receptor expression. Interestingly, CsA treatment had no significant influence on CIK cell viability and the cytotoxic potential against K562. CONCLUSIONS: Our data indicate that if immunosuppressant therapy is indispensable, efficacy of CIK cells is maintained at least short-term, although more frequent dosing might be necessary.
Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition.[Pubmed:27580029]
Nature. 2016 Aug 31;537(7621):544-547.
Mutations of the tricarboxylic acid cycle enzyme fumarate hydratase cause hereditary leiomyomatosis and renal cell cancer. Fumarate hydratase-deficient renal cancers are highly aggressive and metastasize even when small, leading to a very poor clinical outcome. Fumarate, a small molecule metabolite that accumulates in fumarate hydratase-deficient cells, plays a key role in cell transformation, making it a bona fide oncometabolite. Fumarate has been shown to inhibit alpha-ketoglutarate-dependent dioxygenases that are involved in DNA and histone demethylation. However, the link between fumarate accumulation, epigenetic changes, and tumorigenesis is unclear. Here we show that loss of fumarate hydratase and the subsequent accumulation of fumarate in mouse and human cells elicits an epithelial-to-mesenchymal-transition (EMT), a phenotypic switch associated with cancer initiation, invasion, and metastasis. We demonstrate that fumarate inhibits Tet-mediated demethylation of a regulatory region of the antimetastatic miRNA cluster mir-200ba429, leading to the expression of EMT-related transcription factors and enhanced migratory properties. These epigenetic and phenotypic changes are recapitulated by the incubation of fumarate hydratase-proficient cells with cell-permeable fumarate. Loss of fumarate hydratase is associated with suppression of miR-200 and the EMT signature in renal cancer and is associated with poor clinical outcome. These results imply that loss of fumarate hydratase and fumarate accumulation contribute to the aggressive features of fumarate hydratase-deficient tumours.
Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway.[Pubmed:22267202]
J Pharmacol Exp Ther. 2012 Apr;341(1):274-84.
Oxidative stress is central to the pathology of several neurodegenerative diseases, including multiple sclerosis, and therapeutics designed to enhance antioxidant potential could have clinical value. The objective of this study was to characterize the potential direct neuroprotective effects of dimethyl fumarate (DMF) and its primary metabolite monomethyl fumarate (MMF) on cellular resistance to oxidative damage in primary cultures of central nervous system (CNS) cells and further explore the dependence and function of the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathway in this process. Treatment of animals or primary cultures of CNS cells with DMF or MMF resulted in increased nuclear levels of active Nrf2, with subsequent up-regulation of canonical antioxidant target genes. DMF-dependent up-regulation of antioxidant genes in vivo was lost in mice lacking Nrf2 [Nrf2(-/-)]. DMF or MMF treatment increased cellular redox potential, glutathione, ATP levels, and mitochondrial membrane potential in a concentration-dependent manner. Treating astrocytes or neurons with DMF or MMF also significantly improved cell viability after toxic oxidative challenge in a concentration-dependent manner. This effect on viability was lost in cells that had eliminated or reduced Nrf2. These data suggest that DMF and MMF are cytoprotective for neurons and astrocytes against oxidative stress-induced cellular injury and loss, potentially via up-regulation of an Nrf2-dependent antioxidant response. These data also suggest DMF and MMF may function through improving mitochondrial function. The clinical utility of DMF in multiple sclerosis is being explored through phase III trials with BG-12, which is an oral therapeutic containing DMF as the active ingredient.
The psoriasis drug monomethylfumarate is a potent nicotinic acid receptor agonist.[Pubmed:18722346]
Biochem Biophys Res Commun. 2008 Oct 31;375(4):562-5.
Nicotinic acid has been used for several decades to treat dyslipidemia. In mice, the lipid-lowing effect of nicotinic acid is mediated by the Gi coupled receptor PUMA-G. In humans, high (GPR109A) and low (GPR109B) affinity nicotinic acid receptors have been characterized. Here we identify monomethylfumarate as a GPR109A agonist. Monomethylfumarate is the active metabolite of the psoriasis drug Fumaderm. We show that monomethylfumarate activates GPR109A in a calcium based aequorin assay, cAMP assay and demonstrate competitive binding with nicotinic acid. We show that GPR109A is highly expressed in neutrophils and epidermal keratinocytes, and that its expression is increased in human psoriatic lesions. Our findings provide evidence that GPR109A is a target for the drug Fumaderm and suggest that niacin should be investigated to treat psoriasis in addition to its role in treating lipid disorders.
Antihepatotoxic activity of monomethyl fumarate isolated from Fumaria indica.[Pubmed:9613834]
J Ethnopharmacol. 1998 Apr;60(3):207-13.
Monomethyl fumarate, isolated for the first time from the methanolic extract of the whole plant of Fumaria indica, was characterised and screened for its antihepatotoxic activity in albino rats. The compound showed significant (P < 0.01) antihepatotoxic activity against thioacetamide in vitro, and against hepatotoxicities induced by carbon tetrachloride, paracetamol and rifampicin in vivo to an extent almost similar to that of silymarin, a known antihepatotoxic agent.


