Communic acidCAS# 2761-77-5 |
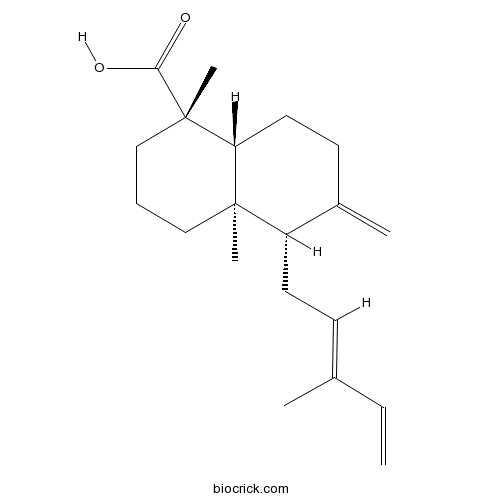
- 4-Epicommunic acid
Catalog No.:BCN4382
CAS No.:83945-57-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2761-77-5 | SDF | Download SDF |
| PubChem ID | 637125 | Appearance | Powder |
| Formula | C20H30O2 | M.Wt | 302.5 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Synonyms | 10178-32-2;trans-Communic acid | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1S,4aR,5S,8aR)-1,4a-dimethyl-6-methylidene-5-[(2E)-3-methylpenta-2,4-dienyl]-3,4,5,7,8,8a-hexahydro-2H-naphthalene-1-carboxylic acid | ||
| SMILES | CC(=CCC1C(=C)CCC2C1(CCCC2(C)C(=O)O)C)C=C | ||
| Standard InChIKey | YGBZFOQXPOGACY-VWVSFFKRSA-N | ||
| Standard InChI | InChI=1S/C20H30O2/c1-6-14(2)8-10-16-15(3)9-11-17-19(16,4)12-7-13-20(17,5)18(21)22/h6,8,16-17H,1,3,7,9-13H2,2,4-5H3,(H,21,22)/b14-8+/t16-,17+,19+,20-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. trans-Communic acid has potential to be utilized as anti-wrinkling agents and cosmetic ingredients, as they suppress UVB-induced MMP-1 expression. 2. Communic acid has antibacterial activity, including against mycobacterial. 3. trans-Communic acid exhibits protective effects against UVB-induced skin aging by suppressing UVB-induced MMP-1 expression. 4. trans-Communic acid shows considerable cytotoxicity against four human cancer cell lines in vitro. 5. trans-Communic acid shows good activity against Mycobacterium aurum (MIC of 13.2 microM). |
| Targets | AP-1 | JNK | Akt | MAPK | PI3K | MMP(e.g.TIMP) | Antifection |

Communic acid Dilution Calculator

Communic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3058 mL | 16.5289 mL | 33.0579 mL | 66.1157 mL | 82.6446 mL |
| 5 mM | 0.6612 mL | 3.3058 mL | 6.6116 mL | 13.2231 mL | 16.5289 mL |
| 10 mM | 0.3306 mL | 1.6529 mL | 3.3058 mL | 6.6116 mL | 8.2645 mL |
| 50 mM | 0.0661 mL | 0.3306 mL | 0.6612 mL | 1.3223 mL | 1.6529 mL |
| 100 mM | 0.0331 mL | 0.1653 mL | 0.3306 mL | 0.6612 mL | 0.8264 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- MMF
Catalog No.:BCC7941
CAS No.:2756-87-8
- Baccatin III
Catalog No.:BCN5165
CAS No.:27548-93-2
- MS 245 oxalate
Catalog No.:BCC6127
CAS No.:275363-58-1
- H-Gly-OtBu.HCl
Catalog No.:BCC2952
CAS No.:27532-96-3
- Feretoside
Catalog No.:BCN5164
CAS No.:27530-67-2
- Gedunin
Catalog No.:BCC7676
CAS No.:2753-30-2
- H-Cha-OH
Catalog No.:BCC2663
CAS No.:27527-05-5
- Gambogic acid
Catalog No.:BCN2318
CAS No.:2752-65-0
- Mesaconitine
Catalog No.:BCN5987
CAS No.:2752-64-9
- O-Methylpallidine
Catalog No.:BCN3916
CAS No.:27510-33-4
- Physalin C
Catalog No.:BCN7918
CAS No.:27503-33-9
- Protostemonine
Catalog No.:BCN8172
CAS No.:27495-40-5
- Danaidal
Catalog No.:BCN1965
CAS No.:27628-46-2
- Rhodexin B
Catalog No.:BCC8246
CAS No.:2763-20-4
- Muscimol
Catalog No.:BCC6593
CAS No.:2763-96-4
- 15-Nonacosanol
Catalog No.:BCC8439
CAS No.:2764-81-0
- 5-Isoquinolinesulfonic acid
Catalog No.:BCC8749
CAS No.:27655-40-9
- Boc-Ser-OMe
Catalog No.:BCC3441
CAS No.:2766-43-0
- Cyanidin-3-O-galactoside chloride
Catalog No.:BCN3022
CAS No.:27661-36-5
- Leucoside
Catalog No.:BCN5167
CAS No.:27661-51-4
- Akt/SKG Substrate Peptide
Catalog No.:BCC5748
CAS No.:276680-69-4
- WAY 161503 hydrochloride
Catalog No.:BCC7179
CAS No.:276695-22-8
- Cryptomoscatone D2
Catalog No.:BCN7203
CAS No.:276856-55-4
- 7-Megastigmene-3,5,6,9-tetraol
Catalog No.:BCN5168
CAS No.:276870-26-9
A new lignan glycoside from Juniperus rigida.[Pubmed:22210029]
Arch Pharm Res. 2011 Dec;34(12):2043-9.
A new lignan glycoside, named juniperigiside (1) was isolated from the CHCl(3) soluble fraction of the MeOH extract of stems and leaves of Juniperus rigida S.et Z. Compound 1 was identified by 1D- and 2D-NMR spectroscopy as well as CD analysis as (2R,3S)-2,3-dihydro-7-methoxy-2-(4'-hydroxy-3'-methoxyphenyl)-3-hydroxymethyl-5-b enzofuranpropanol 4'-O-(3-O-methyl)-alpha-L-rhamnopyranoside. Five known lignans, icariside E4 (2), desoxypodophyllotoxin (3), savinin (4), thujastandin (5), and (-)-nortrachelogenin (6) in addition to five known labdane diterpenes including trans-Communic acid (7), 13-epi-torulosal (8), 13-epi-cupressic acid (9), imbricatoric acid (10), and isocupressic acid (11) were also isolated and their structures were characterized by comparing their spectroscopic data with those in the literature. All compounds were isolated for the first time from this plant, and 5 and 6 were first reported from the genus Juniperus. The isolated compounds were tested for cytotoxicity against four human tumor cell lines in vitro using a Sulforhodamin B bioassay. Compounds 3, 4, 7, and 8 showed considerable cytotoxicity against four human cancer cell lines in vitro.
Antimycobacterial terpenoids from Juniperus communis L. (Cuppressaceae).[Pubmed:19755141]
J Ethnopharmacol. 2009 Dec 10;126(3):500-5.
ETHNOPHARMACOLOGICAL RELEVANCE: Juniperus communis is a plant which has been reported as a traditional cure for tuberculosis (TB) and other respiratory diseases. AIM OF THE STUDY: The aim of this study was to isolate and identify the constituents responsible for the activity of the n-hexane extract of Juniperus communis roots against Mycobacterium tuberculosis H(37)Rv and Juniperus communis aerial parts against Mycobacterium aurum. Subsequently, it was to evaluate the activity of the pure isolated compounds against (i) drug-resistant Mycobacterium tuberculosis variants, (ii) non-replicating Mycobacterium tuberculosis and (iii) a range of non-tuberculous mycobacteria (NTM). MATERIALS AND METHODS: The antimycobacterial activity of Juniperus communis extracts, fractions and constituents was determined against Mycobacterium tuberculosis H(37)Rv, and against rifampicin-, isoniazid-, streptomycin- and moxifloxacin-resistant variants, using the microplate broth Alamar Blue assay (MABA) method. Isolated constituents were tested against non-replicating Mycobacterium tuberculosis H(37)Rv, using the low oxygen recovery assay (LORA), and against NTM (Mycobacterium aurum, Mycobacterium phlei, Mycobacterium fortuitum and Mycobacterium smegmatis), using a broth microdilution method. Cytotoxicity studies were performed using mammalian Vero cells. RESULTS: The antimycobacterial activity of Juniperus communis was attributed to a sesquiterpene identified as longifolene (1) and two diterpenes, characterised as totarol (2) and trans-Communic acid (3). All compounds were identified following analysis of their spectroscopic data (1D- and 2D-NMR, MS) and by comparison with the literature and commercial authentic standards when available. Revised assignments for 3 are reported. Totarol showed the best activity against Mycobacterium tuberculosis H(37)Rv (MIC of 73.7 microM). It was also most active against the isoniazid-, streptomycin-, and moxifloxacin-resistant variants (MIC of 38.4, 83.4 and 60 microM, respectively). Longifolene and totarol were most active against the rifampicin-resistant variant (MICs of 24 and 20.2 microM, respectively). Totarol showed the best activity in the LORA assay (MIC of 81.3 microM) and against all NTM species (MICs in the range of 7-14 microM). Trans-Communic acid showed good activity against Mycobacterium aurum (MIC of 13.2 microM). The low selectivity indices (SI) obtained following cytotoxicity studies indicated that the isolated terpenoids were relatively toxic towards mammalian cells. This is the first report of the isolation of (1) and (2) from Juniperus communis roots, and of (3) from the aerial parts. The antimycobacterial activity of (1) and (3), and the activity of (2) against Mycobacterium aurum, Mycobacterium fortuitum and Mycobacterium phlei, is reported for the first time. The effect of totarol on drug-resistant variants and non-replicating Mycobacterium tuberculosis has never been published. CONCLUSIONS: The presence of antimycobacterial terpenoids in Juniperus communis aerial parts and roots justifies, to some extent, the ethnomedicinal use of this species as a traditional anti-TB remedy.
Antiparasitic, nematicidal and antifouling constituents from Juniperus berries.[Pubmed:19067375]
Phytother Res. 2008 Dec;22(12):1570-6.
A bioassay-guided fractionation of Juniperus procera berries yielded antiparasitic, nematicidal and antifouling constituents, including a wide range of known abietane, pimarane and labdane diterpenes. Among these, abieta-7,13-diene (1) demonstrated in vitro antimalarial activity against Plasmodium falciparum D6 and W2 strains (IC(50) = 1.9 and 2.0 microg/mL, respectively), while totarol (6), ferruginol (7) and 7beta-hydroxyabieta-8,13-diene-11,12-dione (8) inhibited Leishmania donovani promastigotes with IC(50) values of 3.5-4.6 microg/mL. In addition, totarol demonstrated nematicidal and antifouling activities against Caenorhabditis elegans and Artemia salina at a concentration of 80 microg/mL and 1 microg/mL, respectively. The resinous exudate of J. virginiana afforded known antibacterial E-Communic acid (4) and 4-epi-abietic acid (5), while the volatile oil from its trunk wood revealed large quantities of cedrol (9). Using GC/MS, the two known abietanes totarol (6) and ferruginol (7) were identified from the berries of J. procera, J. excelsa and J. phoenicea.
Anti-mycobacterial natural products from the Canadian medicinal plant Juniperus communis.[Pubmed:22877928]
J Ethnopharmacol. 2012 Sep 28;143(2):695-700.
ETHNOPHARMACOLOGICAL RELEVANCE: Common juniper, Juniperus communis, is amongst the plants most frequently used by the indigenous peoples of North America for medicinal purposes. The First Nations of the Canadian Maritimes use infusions of juniper primarily as a tonic and for the treatment of tuberculosis. Previous investigations of extracts derived from the aerial parts of J. communis have shown it to possess anti-mycobacterial activity. The aim of the study is to isolate and identify anti-mycobacterial constituents from the aerial parts of J. communis. MATERIALS AND METHODS: Methanolic extracts of J. communis needles and branches were subjected to bioassay guided fractionation using the microplate resazurin assay (MRA) to assess inhibitory activity against Mycobacterium tuberculosis strain H37Ra. The anti-mycobacterial constituents were identified by NMR, MS and polarimetry. RESULTS: The diterpenes isocupressic acid and Communic acid and the aryltetralin lignan deoxypodophyllotoxin were isolated from the J. communis extract. Isocupressic acid and Communic acid (isolated as an inseparable 3:2 mixture of cis and trans isomers) displayed MICs of 78 muM and 31 muM and IC(50)s of 46 muM and 15 muM against M. tuberculosis H37Ra respectively. Deoxypodophyllotoxin was less active, with a MIC of 1004 muM and an IC(50) of 287 muM. CONCLUSIONS: Isocupressic acid, Communic acid and deoxypodophyllotoxin were identified as the principal constituents responsible for the anti-mycobacterial activity of the aerial parts of J. communis. Although further research will be required to evaluate the relative activities of the two Communic acid isomers, this work validates an ethnopharmacological use of this plant by Canadian First Nations and Native American communities.
Brown Pine Leaf Extract and Its Active Component Trans-Communic Acid Inhibit UVB-Induced MMP-1 Expression by Targeting PI3K.[Pubmed:26066652]
PLoS One. 2015 Jun 11;10(6):e0128365.
Japanese red pine (Pinus densiflora) is widely present in China, Japan, and Korea. Its green pine leaves have traditionally been used as a food as well as a coloring agent. After being shed, pine leaves change their color from green to brown within two years, and although the brown pine leaves are abundantly available, their value has not been closely assessed. In this study, we investigated the potential anti-photoaging properties of brown pine leaves for skin. Brown pine leaf extract (BPLE) inhibited UVB-induced matrix metalloproteinase-1 (MMP-1) expression to a greater extent than pine leaf extract (PLE) in human keratinocytes and a human skin equivalent model. HPLC analysis revealed that the quantity of trans-Communic acid (TCA) and dehydroabietic acid (DAA) significantly increases when the pine leaf color changes from green to brown. BPLE and TCA elicited reductions in UVB-induced MMP-1 mRNA expression and activator protein-1 (AP-1) transactivation by reducing DNA binding activity of phospho-c-Jun, c-fos and Fra-1. BPLE and TCA also inhibited UVB-induced Akt phosphorylation, but not mitogen activated protein kinase (MAPK), known regulators of AP-1 transactivation. We additionally found that BPLE and TCA inhibited phosphoinositide 3-kinase (PI3K), the upstream kinase of Akt, in vitro. In summary, both BPLE and its active component TCA exhibit protective effects against UVB-induced skin aging. Taken together, these findings underline the potential for BPLE and TCA to be utilized as anti-wrinkling agents and cosmetic ingredients, as they suppress UVB-induced MMP-1 expression.


