MS 245 oxalateHigh affinity 5-HT6 antagonist CAS# 275363-58-1 |
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- BMS-345541
Catalog No.:BCC1423
CAS No.:547757-23-3
- Bay 65-1942 free base
Catalog No.:BCC1408
CAS No.:600734-02-9
- Bay 65-1942 HCl salt
Catalog No.:BCC1409
CAS No.:600734-06-3
- Bay 65-1942 R form
Catalog No.:BCC1410
CAS No.:758683-21-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 275363-58-1 | SDF | Download SDF |
| PubChem ID | 6918541 | Appearance | Powder |
| Formula | C21H24N2O7S | M.Wt | 448.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO | ||
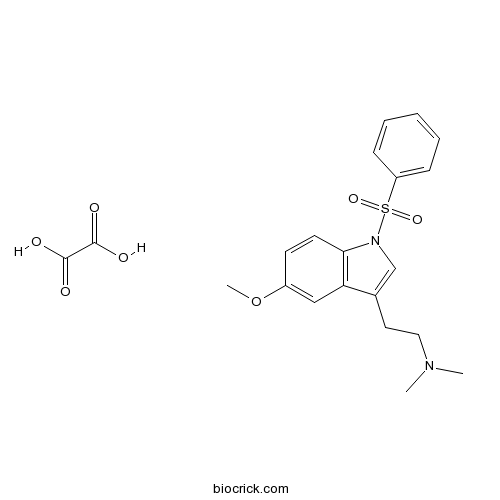
| Chemical Name | 2-[1-(benzenesulfonyl)-5-methoxyindol-3-yl]-N,N-dimethylethanamine;oxalic acid | ||
| SMILES | CN(C)CCC1=CN(C2=C1C=C(C=C2)OC)S(=O)(=O)C3=CC=CC=C3.C(=O)(C(=O)O)O | ||
| Standard InChIKey | BLSWAEGCRSFTJG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H22N2O3S.C2H2O4/c1-20(2)12-11-15-14-21(19-10-9-16(24-3)13-18(15)19)25(22,23)17-7-5-4-6-8-17;3-1(4)2(5)6/h4-10,13-14H,11-12H2,1-3H3;(H,3,4)(H,5,6) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | High affinity 5-HT6 antagonist (Ki = 2.1 nM). Potentiates the hypolocomotor actions of (-)-nicotine in mice. |

MS 245 oxalate Dilution Calculator

MS 245 oxalate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2297 mL | 11.1485 mL | 22.297 mL | 44.5941 mL | 55.7426 mL |
| 5 mM | 0.4459 mL | 2.2297 mL | 4.4594 mL | 8.9188 mL | 11.1485 mL |
| 10 mM | 0.223 mL | 1.1149 mL | 2.2297 mL | 4.4594 mL | 5.5743 mL |
| 50 mM | 0.0446 mL | 0.223 mL | 0.4459 mL | 0.8919 mL | 1.1149 mL |
| 100 mM | 0.0223 mL | 0.1115 mL | 0.223 mL | 0.4459 mL | 0.5574 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- H-Gly-OtBu.HCl
Catalog No.:BCC2952
CAS No.:27532-96-3
- Feretoside
Catalog No.:BCN5164
CAS No.:27530-67-2
- Gedunin
Catalog No.:BCC7676
CAS No.:2753-30-2
- H-Cha-OH
Catalog No.:BCC2663
CAS No.:27527-05-5
- Gambogic acid
Catalog No.:BCN2318
CAS No.:2752-65-0
- Mesaconitine
Catalog No.:BCN5987
CAS No.:2752-64-9
- O-Methylpallidine
Catalog No.:BCN3916
CAS No.:27510-33-4
- Physalin C
Catalog No.:BCN7918
CAS No.:27503-33-9
- Protostemonine
Catalog No.:BCN8172
CAS No.:27495-40-5
- Vildagliptin (LAF-237)
Catalog No.:BCC2112
CAS No.:274901-16-5
- 2-O-Acetyltutin
Catalog No.:BCN5163
CAS No.:2749-28-2
- L-Ala-ol
Catalog No.:BCC2590
CAS No.:2749-11-3
- Baccatin III
Catalog No.:BCN5165
CAS No.:27548-93-2
- MMF
Catalog No.:BCC7941
CAS No.:2756-87-8
- Communic acid
Catalog No.:BCN5166
CAS No.:2761-77-5
- Danaidal
Catalog No.:BCN1965
CAS No.:27628-46-2
- Rhodexin B
Catalog No.:BCC8246
CAS No.:2763-20-4
- Muscimol
Catalog No.:BCC6593
CAS No.:2763-96-4
- 15-Nonacosanol
Catalog No.:BCC8439
CAS No.:2764-81-0
- 5-Isoquinolinesulfonic acid
Catalog No.:BCC8749
CAS No.:27655-40-9
- Boc-Ser-OMe
Catalog No.:BCC3441
CAS No.:2766-43-0
- Cyanidin-3-O-galactoside chloride
Catalog No.:BCN3022
CAS No.:27661-36-5
- Leucoside
Catalog No.:BCN5167
CAS No.:27661-51-4
- Akt/SKG Substrate Peptide
Catalog No.:BCC5748
CAS No.:276680-69-4
Binding of serotonin and N1-benzenesulfonyltryptamine-related analogs at human 5-HT6 serotonin receptors: receptor modeling studies.[Pubmed:18201064]
J Med Chem. 2008 Feb 14;51(3):603-11.
A population of 100 graphics models of the human 5-HT6 serotonin receptor was constructed based on the structure of bovine rhodopsin. The endogenous tryptamine-based agonist serotonin (5-HT; 1) and the benzenesulfonyl-containing tryptamine-derived 5-HT6 receptor antagonist MS-245 (4a) were automatically docked with each of the 100 receptor models using a genetic algorithm approach. Similar studies were conducted with the more selective 5-HT6 receptor agonist EMDT (5) and optical isomers of EMDT-related analog 8, as well as with optical isomers of MS-245 (4a)-related and benzenesulfonyl-containing pyrrolidine 6 and aminotetralin 7. Although associated with the same general aromatic/hydrophobic binding cluster, 5-HT (1) and MS-245 (4a) were found to preferentially bind with distinct receptor conformations, and did so with different binding orientations (i.e., poses). A 5-HT pose/model was found to be common to EMDT (5) and its analogs, whereas that identified for MS-245 (4a) was found common to benzenesulfonyl-containing compounds. Specific amino acid residues were identified that can participate in binding, and evaluation of a sulfenamide analog of MS-245 indicates for the first time that the presence of the sulfonyl oxygen atoms enhances receptor affinity. The results indicate that the presence or absence of an N1-benzenesulfonyl group is a major determinant of the manner in which tryptamine-related agents bind at 5-HT6 serotonin receptors.
Effect of the 5-HT(6) serotonin antagonist MS-245 on the actions of (-)nicotine.[Pubmed:16950502]
Pharmacol Biochem Behav. 2006 Sep;85(1):170-7.
The 5-HT(6) serotonin receptor antagonist MS-245 neither substitutes for nor antagonizes the discriminative stimulus effects of (-)nicotine. However, MS-245 was shown to enhance the potency of (-)nicotine in Sprague-Dawley rats trained to discriminate 0.6 mg/kg of (-)nicotine from saline vehicle in a typical two-lever drug discrimination paradigm such that a combination of MS-245 (5.0 mg/kg) plus the ED(50) dose of (-)nicotine caused the animals to respond as if they had received the training dose of (-)nicotine. MS-245 also potentiated the hypolocomotor actions, but not the antinociceptive effects, of (-)nicotine in mice. The results suggest possible involvement of serotonin-regulated signaling mechanisms in certain behavioral effects of nicotine.
2-Substituted tryptamines: agents with selectivity for 5-HT(6) serotonin receptors.[Pubmed:10715164]
J Med Chem. 2000 Mar 9;43(5):1011-8.
Several 2-alkyl-5-methoxytryptamine analogues were designed and prepared as potential 5-HT(6) serotonin agonists. It was found that 5-HT(6) receptors accommodate small alkyl substituents at the indole 2-position and that the resulting compounds can bind with affinities comparable to that of serotonin. In particular, 2-ethyl-5-methoxy-N, N-dimethyltryptamine (8) binds with high affinity at human 5-HT(6) receptors (K(i) = 16 nM) relative to 5-HT (K(i) = 75 nM) and was a full agonist, at least as potent (8: K(act) = 3.6 nM) as serotonin (K(act) = 5.0 nM), in activating adenylate cyclase. Compound 8 displays modest affinity for several other populations of 5-HT receptors, notably h5-HT(1A) (K(i) = 170 nM), h5-HT(1D) (K(i) = 290 nM), and h5-HT(7) (K(i) = 300 nM) receptors, but is otherwise quite selective. Compound 8 represents the first and most selective 5-HT(6) agonist reported to date. Replacing the 2-ethyl substituent with a phenyl group results in a compound that retains 5-HT(6) receptor affinity (i.e., 10: K(i) = 20 nM) but lacks agonist character. 2-Substituted tryptamines, then, might allow entry to a novel class of 5-HT(6) agonists and antagonists.


