BaldrinalCAS# 18234-46-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 18234-46-3 | SDF | Download SDF |
| PubChem ID | 159846 | Appearance | Powder |
| Formula | C12H10O4 | M.Wt | 218.20 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
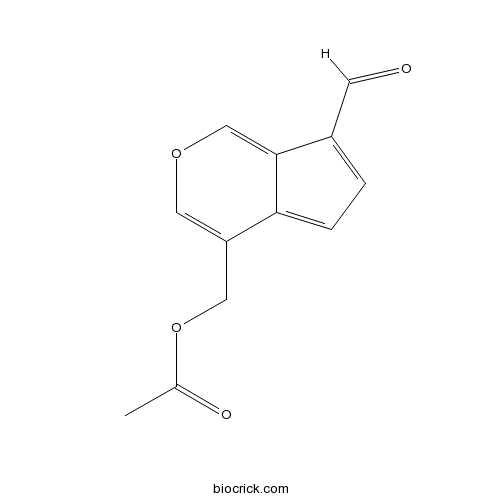
| Chemical Name | (7-formylcyclopenta[c]pyran-4-yl)methyl acetate | ||
| SMILES | CC(=O)OCC1=COC=C2C1=CC=C2C=O | ||
| Standard InChIKey | QIUOVIRIFZOCLL-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Baldrinal shows selective cytotoxicity against metastatic prostate cancer (PC-3M) and colon cancer (HCT-8) cell lines. 2. Baldrinal, valtrate, and acevaltrate are the quality control compounds of Valeriana jatamansi Jones. |

Baldrinal Dilution Calculator

Baldrinal Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.583 mL | 22.9148 mL | 45.8295 mL | 91.659 mL | 114.5738 mL |
| 5 mM | 0.9166 mL | 4.583 mL | 9.1659 mL | 18.3318 mL | 22.9148 mL |
| 10 mM | 0.4583 mL | 2.2915 mL | 4.583 mL | 9.1659 mL | 11.4574 mL |
| 50 mM | 0.0917 mL | 0.4583 mL | 0.9166 mL | 1.8332 mL | 2.2915 mL |
| 100 mM | 0.0458 mL | 0.2291 mL | 0.4583 mL | 0.9166 mL | 1.1457 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Antibiotic 2158
Catalog No.:BCN1825
CAS No.:182320-34-9
- Antibiotic ZG 1494alpha
Catalog No.:BCN1850
CAS No.:182320-33-8
- Synthalin sulfate
Catalog No.:BCC6730
CAS No.:182285-12-7
- Lirioprolioside B
Catalog No.:BCN2740
CAS No.:182284-68-0
- Clausine I
Catalog No.:BCN4687
CAS No.:182261-94-5
- 3,4-seco-Olean-12-en-4-ol-3,28-dioic acid
Catalog No.:BCN7151
CAS No.:182249-69-0
- Nyssoside
Catalog No.:BCN1146
CAS No.:182138-70-1
- Quinovic acid 3-O-(3',4'-O-isopropylidene)-beta-D-fucopyranoside
Catalog No.:BCN1519
CAS No.:182132-59-8
- Nitrosostromelin
Catalog No.:BCN1745
CAS No.:182064-61-5
- 6-Angeloyloxyditropan-3-yl itaconate
Catalog No.:BCN1867
CAS No.:182015-05-0
- KB-R7943 mesylate
Catalog No.:BCC1676
CAS No.:182004-65-5
- Meliasendanin D
Catalog No.:BCN7610
CAS No.:1820034-05-6
- Rupatadine Fumarate
Catalog No.:BCC4535
CAS No.:182349-12-8
- Lomitapide
Catalog No.:BCC5570
CAS No.:182431-12-5
- TPMPA
Catalog No.:BCC6903
CAS No.:182485-36-5
- SB 225002
Catalog No.:BCC8077
CAS No.:182498-32-4
- Thalidezine
Catalog No.:BCN7763
CAS No.:18251-36-0
- (9Z,12Z)-N-Benzyloctadeca-9,12-dienamide
Catalog No.:BCN1518
CAS No.:18286-71-0
- Dibritannilactone B
Catalog No.:BCN7776
CAS No.:1829580-18-8
- Valepotriate
Catalog No.:BCN2351
CAS No.:18296-44-1
- Didrovaltrate
Catalog No.:BCN7124
CAS No.:18296-45-2
- 3,4-O-Isopropylidene shikimic acid
Catalog No.:BCN1147
CAS No.:183075-03-8
- Fabiatrin
Catalog No.:BCN2920
CAS No.:18309-73-4
- Cabazitaxel
Catalog No.:BCC4966
CAS No.:183133-96-2
Flexible and powerful strategy for qualitative and quantitative analysis of valepotriates in Valeriana jatamansi Jones using high-performance liquid chromatography with linear ion trap Orbitrap mass spectrometry.[Pubmed:28296152]
J Sep Sci. 2017 May;40(9):1906-1919.
Valeriana jatamansi Jones is an important medicinal plant and its quality is closely related to its region of origin. In the current study, we utilized a flexible and powerful strategy for comprehensive evaluation of the quality diversity for 15 regions in China. The method was based on a hybrid linear ion trap-Orbitrap mass spectrometry platform. For structure characterization, fragmentation patterns were detected by analyzing a series of standard compounds using data dependent multistage mass spectrometry acquisition. A fragment ion database for valepotriates was established, and the acquired data were high throughput filtered by fragment ion search for compound identification. For quantitative purposes, we normalized the mass spectrometry data of 15 samples using SIEVE 2.0 and the differences in composition were analyzed using principal component analysis combined with hierarchical clustering analysis. The results identified a total of 92 compounds from Valeriana jatamansi Jones. Samples from Dali, Kunming, and Baoshan have better qualities and concentrations of the main active constituents. To verify our strategy, we compared the valtrate, acevaltrate, and Baldrinal contents using high-performance liquid chromatography with diode array detector. We developed and validated a comprehensive qualitative and quantitative analytical method to achieve quality control of Valeriana jatamansi Jones.
Three decomposition products of valepotriates from Valeriana jatamansi and their cytotoxic activity.[Pubmed:25971678]
J Asian Nat Prod Res. 2015 May;17(5):455-61.
Three new decomposition products of valepotriates, valtrals A-C (1-3), and two known products, Baldrinal and homoBaldrinal, are formed during the isolation procedure of the ethanol extract of the whole plants of Valeriana jatamansi. Their structures were determined by spectroscopic methods including IR, MS, 1D, and 2D NMR experiments. Compounds 1-3 showed selective cytotoxicity against metastatic prostate cancer (PC-3M) and colon cancer (HCT-8) cell lines.
11-Methoxyviburtinal, a new iridoid from Valeriana jatamansi.[Pubmed:16276973]
Arch Pharm Res. 2005 Oct;28(10):1161-3.
Five compounds of iridoids, lignan and phenylpropanoid glycosides were isolated from the roots of Valeriana jatamansi by column chromatography. Their structures were elucidated as 11-methoxyviburtinal (1), Baldrinal (2), prinsepiol-4-omicron-beta-D-glucoside (3), coniferin (4), and hexacosanic acid (5) by spectroscopic analysis. 11-Methoxyviburtinal was a new compound, and others were isolated from the plant for the first time.


