TPMPACAS# 182485-36-5 |
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- BMS-345541
Catalog No.:BCC1423
CAS No.:547757-23-3
- Bay 65-1942 free base
Catalog No.:BCC1408
CAS No.:600734-02-9
- Bay 65-1942 HCl salt
Catalog No.:BCC1409
CAS No.:600734-06-3
- Bay 65-1942 R form
Catalog No.:BCC1410
CAS No.:758683-21-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 182485-36-5 | SDF | Download SDF |
| PubChem ID | 5521 | Appearance | Powder |
| Formula | C6H12NO2P | M.Wt | 161.14 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
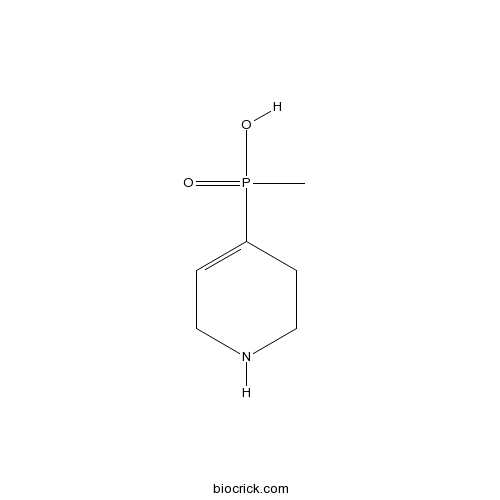
| Chemical Name | (1,2,5,6-Tetrahydropyridin-4-yl)met | ||
| SMILES | CP(=O)(C1=CCNCC1)O | ||
| Standard InChIKey | MFUKVPOVVKKLRQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H12NO2P/c1-10(8,9)6-2-4-7-5-3-6/h2,7H,3-5H2,1H3,(H,8,9) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A selective, competitive GABAA-ρ antagonist with only minimal effects on GABAA and GABAB receptors (Kb values are 2.1 μM (antagonist), 320 μM (antagonist) and EC50 ~ 500 μM (weak agonist) respectively). Displays 8-fold selectivity for human recombinant ρ1 receptors over ρ2 receptors. |

TPMPA Dilution Calculator

TPMPA Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.2058 mL | 31.0289 mL | 62.0578 mL | 124.1157 mL | 155.1446 mL |
| 5 mM | 1.2412 mL | 6.2058 mL | 12.4116 mL | 24.8231 mL | 31.0289 mL |
| 10 mM | 0.6206 mL | 3.1029 mL | 6.2058 mL | 12.4116 mL | 15.5145 mL |
| 50 mM | 0.1241 mL | 0.6206 mL | 1.2412 mL | 2.4823 mL | 3.1029 mL |
| 100 mM | 0.0621 mL | 0.3103 mL | 0.6206 mL | 1.2412 mL | 1.5514 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lomitapide
Catalog No.:BCC5570
CAS No.:182431-12-5
- Rupatadine Fumarate
Catalog No.:BCC4535
CAS No.:182349-12-8
- Baldrinal
Catalog No.:BCN2667
CAS No.:18234-46-3
- Antibiotic 2158
Catalog No.:BCN1825
CAS No.:182320-34-9
- Antibiotic ZG 1494alpha
Catalog No.:BCN1850
CAS No.:182320-33-8
- Synthalin sulfate
Catalog No.:BCC6730
CAS No.:182285-12-7
- Lirioprolioside B
Catalog No.:BCN2740
CAS No.:182284-68-0
- Clausine I
Catalog No.:BCN4687
CAS No.:182261-94-5
- 3,4-seco-Olean-12-en-4-ol-3,28-dioic acid
Catalog No.:BCN7151
CAS No.:182249-69-0
- Nyssoside
Catalog No.:BCN1146
CAS No.:182138-70-1
- Quinovic acid 3-O-(3',4'-O-isopropylidene)-beta-D-fucopyranoside
Catalog No.:BCN1519
CAS No.:182132-59-8
- Nitrosostromelin
Catalog No.:BCN1745
CAS No.:182064-61-5
- SB 225002
Catalog No.:BCC8077
CAS No.:182498-32-4
- Thalidezine
Catalog No.:BCN7763
CAS No.:18251-36-0
- (9Z,12Z)-N-Benzyloctadeca-9,12-dienamide
Catalog No.:BCN1518
CAS No.:18286-71-0
- Dibritannilactone B
Catalog No.:BCN7776
CAS No.:1829580-18-8
- Valepotriate
Catalog No.:BCN2351
CAS No.:18296-44-1
- Didrovaltrate
Catalog No.:BCN7124
CAS No.:18296-45-2
- 3,4-O-Isopropylidene shikimic acid
Catalog No.:BCN1147
CAS No.:183075-03-8
- Fabiatrin
Catalog No.:BCN2920
CAS No.:18309-73-4
- Cabazitaxel
Catalog No.:BCC4966
CAS No.:183133-96-2
- Guanylin (human)
Catalog No.:BCC7204
CAS No.:183200-12-6
- Tipiracil hydrochloride
Catalog No.:BCC2001
CAS No.:183204-72-0
- 1,7-Dihydroxy-2,3-methylenedioxyxanthone
Catalog No.:BCN7543
CAS No.:183210-63-1
GABArho selective antagonist TPMPA partially inhibits GABA-mediated currents recorded from neurones and astrocytes in mouse striatum.[Pubmed:27793773]
Neuropharmacology. 2017 Feb;113(Pt A):407-415.
The neostriatum plays a central role in motor coordination where nerve cells operate neuronal inhibition through GABAergic transmission. The neostriatum expresses a wide range of GABA-A subunits, including GABArho1 and rho2 which are restricted to a fraction of GABAergic interneurons and astrocytes. Spontaneous postsynaptic currents (sPSCs) evoked by 4-aminopyridine (4-AP) were recorded from neurones of the dorsal neostriatum, and their frequency was reduced > 50% by the selective GABArho antagonist (1,2,5,6-Tetrahydropyridine-4-yl) methylphosphinic acid (TPMPA). Additionally, we recorded GABA evoked currents from astrocytes in vitro and in situ. Astrocytes in vitro showed modulation by pentobarbital and desensitization upon consecutive applications of GABA. However, modulation by pentobarbital was absent and no significant desensitization was detected from astrocytes in situ. Moreover, TPMPA-sensitive GABA-currents that were insensitive to bicuculline were also recorded from astrocytes in situ, consistent with our previous study where GABArho expression was demonstrated. Finally, we assessed the mRNA expression of GABArho3, through different stages of postnatal development; double immunofluorescence disclosed GABArho3 expression in calretinin-positive interneurons as well as in astrocytes (>70%). These results add new information about the participation of GABArho subunits in neostriatal interneurons and astrocytes.
Inhibition of form-deprivation myopia by a GABAAOr receptor antagonist, (1,2,5,6-tetrahydropyridin-4-yl) methylphosphinic acid (TPMPA), in guinea pigs.[Pubmed:25120102]
Graefes Arch Clin Exp Ophthalmol. 2014 Dec;252(12):1939-46.
PURPOSE: To investigate the effects of the relatively selective GABAAOr receptor antagonist (1,2,5,6-tetrahydropyridin-4-yl) methylphosphinic acid (TPMPA) on form-deprivation myopia (FDM) in guinea pigs. METHODS: A diffuser was applied monocularly to 30 guinea pigs from day 10 to 21. The animals were randomized to one of five treatment groups. The deprived eye received daily sub-conjunctival injections of 100 mul TPMPA at a concentration of (i) 0.03 %, ( ii) 0.3 %, or (iii) 1 %, a fourth group (iv) received saline injections, and another (v) no injections. The fellow eye was left untreated. An additional group received no treatment to either eye. Prior to and at the end of the treatment period, refraction and ocular biometry were performed. RESULTS: Visual deprivation produced relative myopia in all groups (treated versus untreated eyes, P < 0.05). The amount of myopia was significantly affected by the drug treatment (one-way ANOVA, P < 0.0001); myopia was less in deprived eyes receiving either 0.3 % or 1 % TPMPA (saline = -4.38 +/- 0.57D, 0.3 % TPMPA = -3.00 +/- 0.48D, P < 0.01; 1 % TPMPA = -0.88 +/- 0.51D, P < 0.001). The degree of axial elongation was correspondingly less (saline = 0.13 +/- 0.02 mm, 0.3 % TPMPA = 0.09 +/- 0.01 mm, P < 0.01, 1 % TPMPA = 0.02 +/- 0.01 mm, P < 0.001) as was the VC elongation (saline = 0.08 +/- 0.01 mm, 0.3 % TPMPA = 0.05 +/- 0.01 mm, P < 0.01, 1 % TPMPA = 0.01 +/- 0.01 mm; P < 0.001). ACD and LT were not affected (one-way ANOVA, P > 0.05). One percent TPMPA was more effective at inhibiting myopia than 0.3 % (P < 0.01), and 0.03 % did not appreciably inhibit the myopia (0.03 % TPMPA versus saline, P > 0.05). CONCLUSIONS: Sub-conjunctival injections of TPMPA inhibit FDM in guinea pig models in a dose-dependent manner.
The involvement of GABA-C receptors in paired-pulse depression of inhibitory postsynaptic currents in rat hippocampal CA1 pyramidal neurons.[Pubmed:19100735]
Exp Neurol. 2009 Mar;216(1):243-6.
In rat hippocampal CA1 pyramidal neurons, gamma-aminobutyric acid (GABA) A receptor-mediated inhibitory postsynaptic currents (IPSCs) undergo a paired-pulse depression (PPD) by the second of two pulses, with inter-pulse intervals of 100-2000 ms, applied to the stratum radiatum. While GABA-C receptors are described in the CA1 area, their functional significance is unknown. In this study, the involvement of GABA-C receptors in PPD was examined using an in vitro hippocampal slice preparation. IPSCs evoked by stimulations in stratum radiatum were recorded with patch pipettes from CA1 pyramidal cells. PPD, when induced in the above fashion, was blocked by the GABA-C receptor antagonist (1,2,5,6-Tetrahydropyridin-4-yl) methylphosphinic acid (TPMPA, 10 muM, applied in the superfusing medium). GABA-A and GABA-B receptor-mediated IPSCs, as well as the baclofen-induced suppression of the GABA-A receptor mediated IPSC, were not antagonized by TPMPA (10-20 muM). These results indicate that PPD of the IPSC is mediated by the activation of GABA-C receptors.
GABA(C) receptor antagonists differentiate between human rho1 and rho2 receptors expressed in Xenopus oocytes.[Pubmed:9797041]
Eur J Pharmacol. 1998 Sep 18;357(2-3):227-34.
The selective GABA(C) receptor antagonist, (1,2,5,6-tetrahydropyridin-4-yl)methylphosphinic acid (TPMPA), is eight times more potent against human recombinant p receptors than p2 receptors expressed in Xenopus oocytes. (3-Aminopropyl)methylphosphinic acid (CGP35024), the methylphosphinic acid analogue of GABA, and [(E)-3-aminopropen-1-yl]methylphosphinic acid (CGP44530), an open chain analogue of TPMPA, were five and four times, respectively, more potent as antagonists of p1 receptors than as antagonists of p2 receptors. Isoguvacine was a weak partial agonist at both p1 and p2 receptors with intrinsic activities (calculated as a percentage of the maximum whole cell current produced by a maximum dose of GABA) of 45 and 68%, respectively, of the maximum response produced by GABA. In agreement with other workers, it was found that imidazole-4-acetic acid was a partial agonist at both p1 and p2 receptors, showing higher intrinsic activity at p2 than at p1 receptors. The p1 receptor antagonist, trans-4-amino-2-methylbut-2-enoic acid (2-MeTACA), was a partial agonist at p2 receptors with an intrinsic activity of 34%. 2-MeTACA may be useful in differentiating between homo-oligomeric p1 and p2 receptors in native systems. These studies reveal significant differences in the antagonist profile of human recombinant p1 and p2 GABA(C) receptors.
Design and in vitro pharmacology of a selective gamma-aminobutyric acidC receptor antagonist.[Pubmed:8863850]
Mol Pharmacol. 1996 Oct;50(4):1024-30.
In mammals, receptors for the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) are divided into three pharmacological classes, which are denoted GABAA, GABAB, and GABAC. GABAC receptors are defined by their insensitivity to the GABAA receptor antagonist bicuculline and the GABAB receptor agonist (-)-baclofen. GABAC receptors probably are a heterogeneous group of proteins. The most extensively studied mammalian GABAC receptors are those found in neurons of the outer retina. These receptors are GABA-gated Cl- channels comprised of p subunits, of which there are two subtypes. The physiological functions served by GABAC receptors are largely unknown; to determine the functions, it would be useful to have GABAC-selective ligands. In a previous study, we found that isoguvacine, a GABAA-selective agonist, and 3-aminopropyl-(methyl)phosphinic acid (3-APMPA), a GABAB-selective agonist, show affinity for retinal GABAC receptors. In particular, 3-APMPA is an antagonist with low micromolar potency (Kb approximately 1 microM). Here, we report the synthesis and pharmacological characterization of (1,2,5,6-tetrahydropyridine-4-yl)methylphosphinic acid (TPMPA), a hybrid of isoguvacine and 3-APMPA designed to retain affinity for GABAC receptors but not to interact with GABAA or GABAB receptors. Electrical assays show that TPMPA is a competitive antagonist of cloned human mu 1 GABAC receptors expressed in Xenopus laevis oocytes (Kb approximately 2 microM). TPMPA is > 100-fold weaker as an inhibitor of rat brain GABAA receptors expressed in oocytes (Kb approximately 320 microM) and has only weak agonist activity on GABAB receptors assayed in rat hippocampal slices (EC50 approximately 500 microM). TPMPA should be a useful pharmacological probe with which to investigate GABAC receptor function in the outer retina and in any other areas of the nervous system in which these types of receptor are present.


