Synthalin sulfateCAS# 182285-12-7 |
- StemRegenin 1 (SR1)
Catalog No.:BCC3637
CAS No.:1227633-49-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 182285-12-7 | SDF | Download SDF |
| PubChem ID | 44134960 | Appearance | Powder |
| Formula | C12H30N6O4S | M.Wt | 354.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 5 mM in water | ||
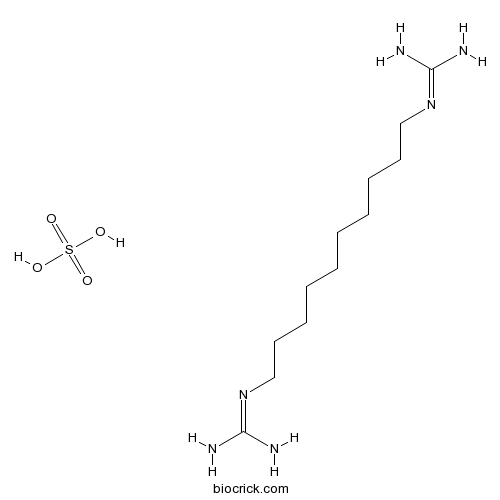
| Chemical Name | 2-[10-(diaminomethylideneamino)decyl]guanidine;sulfuric acid | ||
| SMILES | C(CCCCCN=C(N)N)CCCCN=C(N)N.OS(=O)(=O)O | ||
| Standard InChIKey | MNKRJRJDADVBJJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H28N6.H2O4S/c13-11(14)17-9-7-5-3-1-2-4-6-8-10-18-12(15)16;1-5(2,3)4/h1-10H2,(H4,13,14,17)(H4,15,16,18);(H2,1,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | An effective non-competitive NMDA receptor antagonist in vivo and in vitro; may bind to more than one site, one of which is the polyamine site. |

Synthalin sulfate Dilution Calculator

Synthalin sulfate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8211 mL | 14.1056 mL | 28.2111 mL | 56.4223 mL | 70.5278 mL |
| 5 mM | 0.5642 mL | 2.8211 mL | 5.6422 mL | 11.2845 mL | 14.1056 mL |
| 10 mM | 0.2821 mL | 1.4106 mL | 2.8211 mL | 5.6422 mL | 7.0528 mL |
| 50 mM | 0.0564 mL | 0.2821 mL | 0.5642 mL | 1.1284 mL | 1.4106 mL |
| 100 mM | 0.0282 mL | 0.1411 mL | 0.2821 mL | 0.5642 mL | 0.7053 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lirioprolioside B
Catalog No.:BCN2740
CAS No.:182284-68-0
- Clausine I
Catalog No.:BCN4687
CAS No.:182261-94-5
- 3,4-seco-Olean-12-en-4-ol-3,28-dioic acid
Catalog No.:BCN7151
CAS No.:182249-69-0
- Nyssoside
Catalog No.:BCN1146
CAS No.:182138-70-1
- Quinovic acid 3-O-(3',4'-O-isopropylidene)-beta-D-fucopyranoside
Catalog No.:BCN1519
CAS No.:182132-59-8
- Nitrosostromelin
Catalog No.:BCN1745
CAS No.:182064-61-5
- 6-Angeloyloxyditropan-3-yl itaconate
Catalog No.:BCN1867
CAS No.:182015-05-0
- KB-R7943 mesylate
Catalog No.:BCC1676
CAS No.:182004-65-5
- Meliasendanin D
Catalog No.:BCN7610
CAS No.:1820034-05-6
- BL 1249
Catalog No.:BCC7777
CAS No.:18200-13-0
- Ethyl beta-D-fructofuranoside
Catalog No.:BCN1145
CAS No.:1820-84-4
- Angenomalin
Catalog No.:BCN8246
CAS No.:18199-64-9
- Antibiotic ZG 1494alpha
Catalog No.:BCN1850
CAS No.:182320-33-8
- Antibiotic 2158
Catalog No.:BCN1825
CAS No.:182320-34-9
- Baldrinal
Catalog No.:BCN2667
CAS No.:18234-46-3
- Rupatadine Fumarate
Catalog No.:BCC4535
CAS No.:182349-12-8
- Lomitapide
Catalog No.:BCC5570
CAS No.:182431-12-5
- TPMPA
Catalog No.:BCC6903
CAS No.:182485-36-5
- SB 225002
Catalog No.:BCC8077
CAS No.:182498-32-4
- Thalidezine
Catalog No.:BCN7763
CAS No.:18251-36-0
- (9Z,12Z)-N-Benzyloctadeca-9,12-dienamide
Catalog No.:BCN1518
CAS No.:18286-71-0
- Dibritannilactone B
Catalog No.:BCN7776
CAS No.:1829580-18-8
- Valepotriate
Catalog No.:BCN2351
CAS No.:18296-44-1
- Didrovaltrate
Catalog No.:BCN7124
CAS No.:18296-45-2
Interactions between zinc and spermidine on the N-methyl-D-aspartate receptor complex: clues to the mechanism of action of 1,10-bis(guanidino)decane and pentamidine.[Pubmed:1432693]
J Pharmacol Exp Ther. 1992 Nov;263(2):632-8.
This study investigated the mechanism of action of two novel, noncompetitive antagonists of the N-methyl-D-aspartate (NMDA) receptor using [3H]dizocilpine binding to rat brain membranes. Pentamidine and 1,10(bisguanidino)decane (BG10) inhibited [3H]dizocilpine binding in the concentration range of 1 to 100 microM, as did Zn++ and the competitive polyamine antagonist arcaine. The action of each of these agents was sensitive to the addition of spermidine to the assay. However, only arcaine interacted with spermidine in a competitive fashion. Spermidine decreased the apparent affinity of Zn++ and also increased the Hill slope of the Zn++ inhibition curves. BG10 and pentamidine inhibition of [3H]dizocilpine binding was less sensitive to spermidine. Calcium had similar effects to spermidine on the inhibition of [3H]dizocilpine binding by Zn++, arcaine, pentamidine and GB10, but was less potent than spermidine. Treating membranes with diethylpyrocarbonate, a histidine-modifying reagent, decreased the apparent affinity of Zn++ by more than 4-fold while having very modest effects on the actions of BG10, pentamidine and arcaine. In addition, Zn++ failed to slow the dissociation of [3H]dizocilpine in diethylpyrocarbonate-treated tissue, whereas the action of BG10 and pentamidine was qualitatively unaffected. These data show that the effects of BG10 and pentamidine on the NMDA receptor are complex and may involve more than one binding site for each drug. In addition, this study shows that the action of Zn++ on the NMDA receptor is modulated by polyamines. Finally, the mechanism of action of pentamidine and BG10 cannot be attributed to an action at the Zn++ recognition site.
1,10-bis(guanidino)decane inhibits N-methyl-D-aspartate responses in vitro and in vivo.[Pubmed:1658306]
J Pharmacol Exp Ther. 1991 Nov;259(2):626-32.
This study evaluated the interaction of the arcaine analog 1,10-bis(guanidino)decane (BG10) with the NMDA receptor. BG10 inhibited [3H]dizocilpine binding to well-washed rat brain membranes with an apparent affinity of 1.3 microM. The inhibition was not competitive with respect to glutamate or glycine, but was significantly altered by spermidine. However, unlike arcaine, BG10 slowed the dissociation of [3H]dizocilpine. BG10 also inhibited [3H]glycine binding at similar concentrations. BG10 inhibited N-methyl-D-aspartate (NMDA) and glycine-induced increases in intracellular Ca++ in cultured rat brain neurons monitored using the fluorescent dye, fura-2 (IC50 3 microM). This inhibition was not competitive with NMDA or glycine and could not be reversed by either spermidine or arcaine. BG10 also noncompetitively inhibited NMDA-stimulated cyclic GMP production in cerebellar slices from mouse brain. Finally, BG10 administered i.p. reduced harmaline-stimulated, NMDA-dependent cyclic GMP accumulation in mouse cerebellum in vivo (ED50 12.3 mg/kg). These data demonstrate that BG10 is a novel and effective NMDA receptor antagonist in vitro and in vivo.


