DidrovaltrateCAS# 18296-45-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 18296-45-2 | SDF | Download SDF |
| PubChem ID | 65689 | Appearance | Powder |
| Formula | C22H32O8 | M.Wt | 424.49 |
| Type of Compound | Iridoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
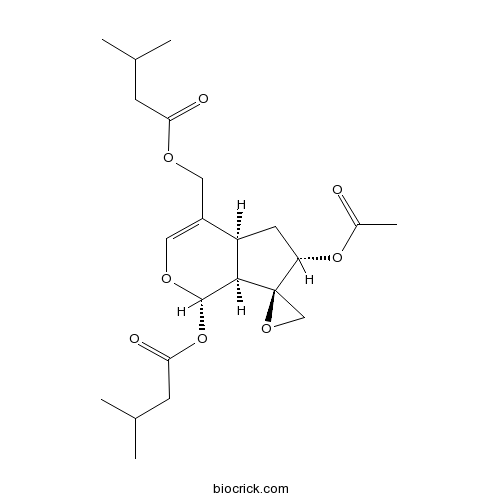
| Chemical Name | [(1S,4aS,6S,7R,7aS)-6-acetyloxy-1-(3-methylbutanoyloxy)spiro[4a,5,6,7a-tetrahydro-1H-cyclopenta[c]pyran-7,2'-oxirane]-4-yl]methyl 3-methylbutanoate | ||
| SMILES | CC(C)CC(=O)OCC1=COC(C2C1CC(C23CO3)OC(=O)C)OC(=O)CC(C)C | ||
| Standard InChIKey | PHHROXLDZHUIGO-PNBTUHDLSA-N | ||
| Standard InChI | InChI=1S/C22H32O8/c1-12(2)6-18(24)26-9-15-10-27-21(30-19(25)7-13(3)4)20-16(15)8-17(29-14(5)23)22(20)11-28-22/h10,12-13,16-17,20-21H,6-9,11H2,1-5H3/t16-,17+,20-,21+,22-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Didrovaltrate blocks I(Ca-L) in a concentration-dependent manner and probably inhibits I(Ca-L) in its inactive state, which may contribute to its cardiovascular effect. 2. Didrovaltrate shows cytotoxic against human cancer cell lines. |
| Targets | Calcium Channel |

Didrovaltrate Dilution Calculator

Didrovaltrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3558 mL | 11.7788 mL | 23.5577 mL | 47.1154 mL | 58.8942 mL |
| 5 mM | 0.4712 mL | 2.3558 mL | 4.7115 mL | 9.4231 mL | 11.7788 mL |
| 10 mM | 0.2356 mL | 1.1779 mL | 2.3558 mL | 4.7115 mL | 5.8894 mL |
| 50 mM | 0.0471 mL | 0.2356 mL | 0.4712 mL | 0.9423 mL | 1.1779 mL |
| 100 mM | 0.0236 mL | 0.1178 mL | 0.2356 mL | 0.4712 mL | 0.5889 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Valepotriate
Catalog No.:BCN2351
CAS No.:18296-44-1
- Dibritannilactone B
Catalog No.:BCN7776
CAS No.:1829580-18-8
- (9Z,12Z)-N-Benzyloctadeca-9,12-dienamide
Catalog No.:BCN1518
CAS No.:18286-71-0
- Thalidezine
Catalog No.:BCN7763
CAS No.:18251-36-0
- SB 225002
Catalog No.:BCC8077
CAS No.:182498-32-4
- TPMPA
Catalog No.:BCC6903
CAS No.:182485-36-5
- Lomitapide
Catalog No.:BCC5570
CAS No.:182431-12-5
- Rupatadine Fumarate
Catalog No.:BCC4535
CAS No.:182349-12-8
- Baldrinal
Catalog No.:BCN2667
CAS No.:18234-46-3
- Antibiotic 2158
Catalog No.:BCN1825
CAS No.:182320-34-9
- Antibiotic ZG 1494alpha
Catalog No.:BCN1850
CAS No.:182320-33-8
- Synthalin sulfate
Catalog No.:BCC6730
CAS No.:182285-12-7
- 3,4-O-Isopropylidene shikimic acid
Catalog No.:BCN1147
CAS No.:183075-03-8
- Fabiatrin
Catalog No.:BCN2920
CAS No.:18309-73-4
- Cabazitaxel
Catalog No.:BCC4966
CAS No.:183133-96-2
- Guanylin (human)
Catalog No.:BCC7204
CAS No.:183200-12-6
- Tipiracil hydrochloride
Catalog No.:BCC2001
CAS No.:183204-72-0
- 1,7-Dihydroxy-2,3-methylenedioxyxanthone
Catalog No.:BCN7543
CAS No.:183210-63-1
- AM251
Catalog No.:BCC4412
CAS No.:183232-66-8
- 2-Acetoxy-3-deacetoxycaesaldekarin E
Catalog No.:BCN7476
CAS No.:18326-06-2
- 5-(1-Piperazinyl)benzofuran-2-carboxamide
Catalog No.:BCC8717
CAS No.:183288-46-2
- Erlotinib Hydrochloride
Catalog No.:BCC3645
CAS No.:183319-69-9
- OSI-420
Catalog No.:BCC4472
CAS No.:183320-51-6
- Erlotinib
Catalog No.:BCC1557
CAS No.:183321-74-6
Cytotoxic potential of valerian constituents and valerian tinctures.[Pubmed:23195845]
Phytomedicine. 1998 May;5(3):219-25.
Underground parts of three Valeriana species, namely V. officinalis L. s.l., V. wallichii DC. (V. jatamansi Jones), and V. edulis Nutt. ex Torr & Gray ssp. procera (H.B.K.) F. G. Meyer (V. mexicana DC.), are used in phytotherapy because of their mild sedative properties. Characteristic constituents of these species, which are regarded also as the active principles, were tested for cytotoxicity against GLC(4), a human small-cell lung cancer cell line, and against COLO 320, a human colorectal cancer cell line, using the microculture tetrazolium (MTT) assay. Valepotriates of the diene type (valtrate, isovaltrate and acevaltrate) displayed the highest cytotoxicity, with IC50 values of 1-6 muM, following continuous incubation. The monoene type valepotriates (Didrovaltrate and isovaleroxyhydroxyDidrovaltrate) were 2- to 3-fold less toxic. Baldrinal and homobaldrinal, decomposition products of valepotriates, were 10- to 30-fold less toxic than their parent compounds. Isovaltral had a higher cytotoxicity than its parent compound isovaltrate. Valerenic acids (valerenic acid, acetoxyvalerenic acid, hydroxyvalerenic acid and methyl valerenate), which are characteristic for V. officinalis, had a low toxicity with IC(50) values between 100 and 200 muM. Freshly prepared and stored tinctures, prepared from roots and rhizomes of the three valerian species, were analysed for valepotriates, baldrinals and valerenic acids, and also tested for cytotoxicity. There was a clear relationship between the valepotriate contents of the freshly prepared tinctures and their toxicity. Upon storage, valepotriates decomposed, which was reflected in a significant reduction of the cytotoxic effect.
Control of development and valepotriate production by auxins in micropropagated Valeriana glechomifolia.[Pubmed:15252693]
Plant Cell Rep. 2004 Oct;23(4):251-5.
Valeriana glechomifolia is a plant species endemic to southern Brazil that accumulates valepotriates, which are terpene derivatives, in all of its organs. Valepotriates are the presumed sedative generic components of the pharmaceutically used species of Valeriana. The influence of various concentrations of the auxins indole-3-acetic acid, indole-3-butyric acid and alpha-naphthaleneacetic acid on the growth of micropropagated V. glechomifolia was investigated under conditions of transient and continuous exposure. Changes in the development of roots and shoots as well as the production of the valepotriates acevaltrate, valtrate and Didrovaltrate (analyzed by high-performance liquid chromatography) were evaluated. The best performance in valepotriate production, growth and survival under ex vitro conditions following plant acclimatization was achieved in the continuous presence of 5.71 microM IAA. When cultured in medium containing IAA plants produced stable levels of valepotriates throughout the entire cultivation period.
Effect of didrovaltrate on I-calcium current in rabbit ventricular myocytes.[Pubmed:23297570]
J Tradit Chin Med. 2012 Sep;32(3):442-5.
OBJECTIVE: To investigate the effect of Didrovaltrate on L-type calcium current (I(Ca-L)) in rabbit ventricular myocytes. METHODS: We used the whole cell patch clamp recording technique. RESULTS: Didrovaltrate at concentrations of 30 microg/ L and 100 microg/L significantly decreased peak I(Ca-L) (I(Ca-Lmax)) from (6.01 +/- 0.48) pA/pF to (3.45 +/- 0.27) pA/pF and (2.16 +/- 0.19) pA/pF (42.6% and 64.1%, n=8, P< 0.01), respectively. Didrovaltrate shifted upwards the current-voltage curves of I(Ca-L) without changing their active, peak and reverse potentials. Didrovaltrate affected the steady-state inactivation of I(Ca-L). The half activation potential (V1/2) was significantly shifted from (-26 +/- 2) to (-36 +/- 3) mV (n=6, P<0.05), with a significant change in the slope factor (k) (from 8.8 +/- 0.8 to 11.1 +/- 0.9, n=6, P<0.05). Didrovaltrate did not affect the activation curve. CONCLUSION: Didrovaltrate blocks I(Ca-L) in a concentration-dependent manner and probably inhibits I(Ca-L) in its inactive state, which may contribute to its cardiovascular effect.


