BenzoguanamineCAS# 91-76-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 91-76-9 | SDF | Download SDF |
| PubChem ID | 7064 | Appearance | Powder |
| Formula | C9H9N5 | M.Wt | 187 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
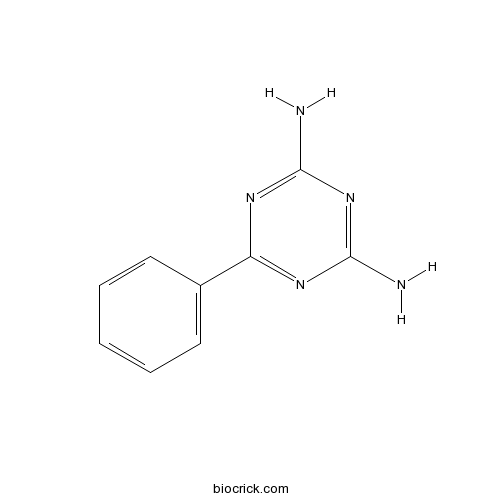
| Chemical Name | 6-phenyl-1,3,5-triazine-2,4-diamine | ||
| SMILES | C1=CC=C(C=C1)C2=NC(=NC(=N2)N)N | ||
| Standard InChIKey | GZVHEAJQGPRDLQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H9N5/c10-8-12-7(13-9(11)14-8)6-4-2-1-3-5-6/h1-5H,(H4,10,11,12,13,14) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Benzoguanamine Dilution Calculator

Benzoguanamine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.3476 mL | 26.738 mL | 53.4759 mL | 106.9519 mL | 133.6898 mL |
| 5 mM | 1.0695 mL | 5.3476 mL | 10.6952 mL | 21.3904 mL | 26.738 mL |
| 10 mM | 0.5348 mL | 2.6738 mL | 5.3476 mL | 10.6952 mL | 13.369 mL |
| 50 mM | 0.107 mL | 0.5348 mL | 1.0695 mL | 2.139 mL | 2.6738 mL |
| 100 mM | 0.0535 mL | 0.2674 mL | 0.5348 mL | 1.0695 mL | 1.3369 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Coumarin
Catalog No.:BCN6309
CAS No.:91-64-5
- Syringol
Catalog No.:BCN3534
CAS No.:91-10-1
- 2,6-Bis(hydroxymethyl)-p-cresol
Catalog No.:BCC8505
CAS No.:91-04-3
- 2-Benzoylpyridine
Catalog No.:BCC8562
CAS No.:91-02-1
- 8-Methoxybonducellin
Catalog No.:BCN4453
CAS No.:90996-27-3
- A 83-01
Catalog No.:BCC1319
CAS No.:909910-43-6
- W146
Catalog No.:BCC7723
CAS No.:909725-61-7
- α-CGRP (human)
Catalog No.:BCC5962
CAS No.:90954-53-3
- Cl-HIBO
Catalog No.:BCC7147
CAS No.:909400-43-7
- Erythrocentauric acid
Catalog No.:BCN7683
CAS No.:90921-13-4
- Broussonin E
Catalog No.:BCN4452
CAS No.:90902-21-9
- M871
Catalog No.:BCC5930
CAS No.:908844-75-7
- N,N'-Bis(acetoacetyl)-o-toluidine
Catalog No.:BCC9062
CAS No.:91-96-3
- Fmoc-Arg-OH
Catalog No.:BCC3039
CAS No.:91000-69-0
- Impurity of Calcipotriol
Catalog No.:BCC5388
CAS No.:910133-69-6
- CGI-1746
Catalog No.:BCC1473
CAS No.:910232-84-7
- RGDS peptide
Catalog No.:BCC7694
CAS No.:91037-65-9
- BAY 60-6583
Catalog No.:BCC6197
CAS No.:910487-58-0
- Danshenol C
Catalog No.:BCN6681
CAS No.:910856-25-6
- 8-Epidiosbulbin E acetate
Catalog No.:BCN7812
CAS No.:91095-48-6
- 3,6,19,23-Tetrahydroxy-12-ursen-28-oic acid
Catalog No.:BCN1310
CAS No.:91095-51-1
- Furowanin A
Catalog No.:BCN4790
CAS No.:911004-72-3
- Isotussilagine
Catalog No.:BCN1985
CAS No.:91108-32-6
- SB 706504
Catalog No.:BCC5615
CAS No.:911110-38-8
Preparation, characterization and molecular modeling studies of the beta-cyclodextrin inclusion complex with benzoguanamine and its analytical application as chemosensor for the selective sensing of Ce(4).[Pubmed:29689512]
Spectrochim Acta A Mol Biomol Spectrosc. 2018 Jul 5;200:212-225.
The inclusion complex of beta-cyclodextrin (beta-CD) with Benzoguanamine (BGA) has been investigated in three states. UV-Visible and fluorescence spectral techniques are used in liquid state. FTIR, NMR and MASS techniques are used in solid state and virtual state studies are done by molecular simulation work. The binding constants for the formation of 1:1 BGA:beta-CD inclusion complex is estimated by UV-Visible and fluorescence spectral techniques. The chemosensory ability of BGA:beta-CD complex was investigated thoroughly for various metal cations and we found the emission of complex showed a linear increase in the intensity for Ce(4+) with the linearity range of 1000muM-2000muM. Sensitivity analysis shows good sensing for Ce(4+) with the LOD of 671muM and LOQ of 2034muM. Our result suggests that the BGA:beta-CD inclusion complex would be promising material for developing solid state sensory device for sensing Ce(4+).
Evaluation of Short-Term and Long-Term Migration Testing from Can Coatings into Food Simulants: Epoxy and Acrylic-Phenolic Coatings.[Pubmed:28282124]
J Agric Food Chem. 2017 Mar 29;65(12):2594-2602.
Traditionally, migration testing during 10 days at 40 degrees C has been considered sufficient and appropriate for simulating the potential migration of substances from food-contact materials into foods. However, some packages, such as food cans, may be stored holding food for extended time periods (years). This study attempts to verify whether common testing conditions accurately estimate long-term migration. Two types of can coatings, epoxy and acrylic-phenolic, were subjected to short-term and long-term migration testing (1 day-1.5 years) using food simulants (water, 3% acetic acid, 50% ethanol, and isooctane) at 40 degrees C. Using HPLC-DAD/CAD, HPLC-MS, UHPLC-HRMS (where HRMS is accurate mass, mass spectrometry), and DART-HRMS, we identified potential migrants before starting the experiment: BPA, BADGE, BADGE derivatives, Benzoguanamine, and other relevant marker compounds. During the experiment using a water-based food simulant, migrants remained stable. Most of the cans in contact with 3% acetic acid did not survive the experimental conditions. Tracked migrants were not detected in isooctane. In the presence of 50% ethanol, the traditional migration test during 10 days at 40 degrees C did not predict migration during long-term storage. These results suggest that migration protocols should be modified to account for long-term storage.
Target and non-target analysis of migrants from PVC-coated cans using UHPLC-Q-Orbitrap MS: evaluation of long-term migration testing.[Pubmed:26744815]
Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2016;33(2):352-63.
A simple, rapid and sensitive method for analyzing multi-target and non-target additives in polyvinyl chloride (PVC) food can coatings using ultra-high-performance liquid chromatography coupled to quadrupole-orbital ion-trap mass spectrometry was developed. This procedure was used to study the behaviour of a cross-linking agent, Benzoguanamine (BGA), two slip agents, oleamide and erucamide, and 18 other commonly used plasticisers including phthalates, adipates, sebacates, acetyl tributyl citrate and epoxidised soybean or linseed oils. This optimised method was used to detect these analytes in food simulants (water and 3% acetic acid) in a long-term migration test of PVC-coated food cans for a period ranging from 1 day to 1.5 years at 40 degrees C. Although very low detection limits (5 ng ml(-1)) were obtained for the majority of compounds, none of the monitored plasticisers and slip agents was detected in simulants extracted from cans over the period of the test. However, the presence of BGA in both aqueous food simulants was confirmed based on high-resolution mass spectrometry, product ion spectra and analysis of a reference standard. The BGA concentration in both simulants continued to increase with storage time: after 1.5 years storage in aqueous food simulants at 40 degrees C, BGA was detected at concentrations up to 84 microg dm(-2). We believe this is the first study describing the long-term migration capacity of BGA from any vinyl coating material intended for use in PVC-coated food cans. Our results may have implications for migration test protocols for food cans that will be stored for extended time periods.
Cu(II) complexes with a sulfonamide derived from benzoguanamine. Oxidative cleavage of DNA in the presence of H2O2 and ascorbate.[Pubmed:15894377]
J Inorg Biochem. 2005 Jul;99(7):1441-8.
Reaction between Benzoguanamine (2,4-diamino-6-phenyl-1,3,5-triazine) and 2-mesitylenesulfonyl chloride leads to formation of a sulfonamide able to form two mononuclear Cu(II) complexes with a CuL(2) stoichiometry. The local environment of the metal cation is a distorted octahedron, with two ligands and two solvent molecules; both complexes crystallize in the monoclinic structure, space group P2(1), with Z=2. In the presence of ascorbate/H(2)O(2,) the two complexes significantly cleavage double-strand pUC18 DNA plasmid. Both complexes exhibit more nuclease efficiency that the copper phenantroline complex. From scavenging reactive oxygen studies we conclude that the hydroxyl radical and a singlet oxygen-like entity, such a peroxide copper complex, are the radical species involved in the DNA damage.
[Solid phase coordination synthesis and characterization of polymimide and Sm ion-under microwave radiation].[Pubmed:12914183]
Guang Pu Xue Yu Guang Pu Fen Xi. 2002 Dec;22(6):990-5.
Solid phase coordination reaction of Sm3+ and the resultant of the imidization of polycondensor of polycondensation and imidization of Benzoguanamine(BGA) and 2, 4-tolylenediisocyanate (TDI) and pyromellitic dianhydride (PMIDA) under microwave irradiation were synthesized and studied. The effect of microwave irradiation time (power), the composition of reactants and the reaction temperature on the yield and Sm content in complexes were studied. The complex was determined by Fourier transform infrared absorption (FTIR), Fourier transform Roman spectrum (FTRS), scanning electric minor (SEM), 13C solid state nuclear magnetic resonance spectrometry and X-ray powder diffraction. The fluorescence intensity was measured by fluorescent emission spectrum and compared with thermal coordination. The magnetic susceptibilities were measured by magnetic curve. The results showed that the complex had not characteristic fluorescence of Sm3+, which illustrated that the first excitation level of Sm3+ and polymer could not match at all. But the complex showed good magnetic property of the ion.
Testing of twenty-one environmental aromatic amines or derivatives for long-term toxicity or carcinogenicity.[Pubmed:84039]
J Environ Pathol Toxicol. 1978 Nov-Dec;2(2):325-56.
Twenty-one aromatic amines or derivatives were tested for long-term toxicity by dietary administration to male Charles River rats and male and female HaM/lCR mice. 2,4-Toluenediamine, o-phenylenediamine, o-toluidine, 2,4,6-trimethylaniline, 2,4,5-trimethylaniline, 2,5-xylidine, and 1-chloro-2-nitrobenzene led to tumors in one or more tissues in all three of these animal models. p-Toluidine, 4-chloro-o-toluidine and 1-chloro-4-nitrobenzene had varying degrees of activity, but in male and female mice only. 4-Chloro-4'-aminodiphenyl ether affected male rats and female mice, but there was no consistent dose response. 2,5-Dimethoxy-4'-aminostilbene led to many tumors in male rats but had only a questionable action in male mice. The effect of 3,3',4,4'tetra-aminobiphenyl in male rats was borderline; in the mice only males showed any response. In male mice 2,4,6-trichloroaniline had a fair degree of activity; tetrafluoro-m-phenylenediamine was somewhat less effective and m-toluidine had only questionable activity. 2,4-Xylidine increased lung tumors in female mice at the higher dose only. Four inactive compounds included m-phenyl-enediamine, 2,4-dinitrochlorobenzene, Benzoguanamine, and dicyclopentadiene dioxide. 2,4,6-Trimethylaniline led to cirrhosis of the liver in rats only.


