8-MethoxybonducellinCAS# 90996-27-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 90996-27-3 | SDF | Download SDF |
| PubChem ID | 73353608 | Appearance | Yellow powder |
| Formula | C18H16O5 | M.Wt | 312.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
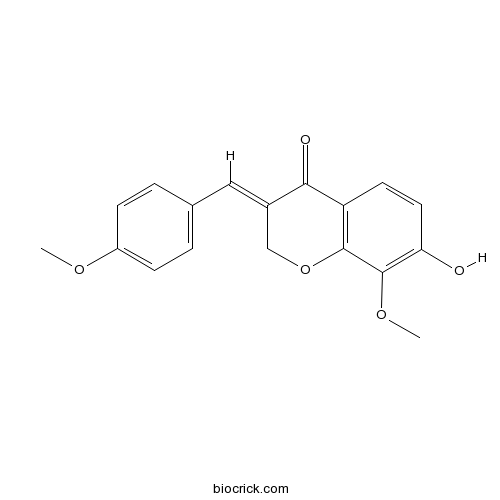
| Chemical Name | (3E)-7-hydroxy-8-methoxy-3-[(4-methoxyphenyl)methylidene]chromen-4-one | ||
| SMILES | COC1=CC=C(C=C1)C=C2COC3=C(C2=O)C=CC(=C3OC)O | ||
| Standard InChIKey | WOXQROZUZURVHX-FMIVXFBMSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 8-Methoxyisobonducellin shows a inhibitory effect on Staphylococcus aureus, Klebsiella Peneumoniae, Beta streptococcus and Aeruginosus bacillus. |
| Targets | Antifection |

8-Methoxybonducellin Dilution Calculator

8-Methoxybonducellin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.202 mL | 16.0102 mL | 32.0205 mL | 64.041 mL | 80.0512 mL |
| 5 mM | 0.6404 mL | 3.202 mL | 6.4041 mL | 12.8082 mL | 16.0102 mL |
| 10 mM | 0.3202 mL | 1.601 mL | 3.202 mL | 6.4041 mL | 8.0051 mL |
| 50 mM | 0.064 mL | 0.3202 mL | 0.6404 mL | 1.2808 mL | 1.601 mL |
| 100 mM | 0.032 mL | 0.1601 mL | 0.3202 mL | 0.6404 mL | 0.8005 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- A 83-01
Catalog No.:BCC1319
CAS No.:909910-43-6
- W146
Catalog No.:BCC7723
CAS No.:909725-61-7
- α-CGRP (human)
Catalog No.:BCC5962
CAS No.:90954-53-3
- Cl-HIBO
Catalog No.:BCC7147
CAS No.:909400-43-7
- Erythrocentauric acid
Catalog No.:BCN7683
CAS No.:90921-13-4
- Broussonin E
Catalog No.:BCN4452
CAS No.:90902-21-9
- M871
Catalog No.:BCC5930
CAS No.:908844-75-7
- α-helical CRF 9-41
Catalog No.:BCC5727
CAS No.:90880-23-2
- Aprotinin
Catalog No.:BCC1220
CAS No.:9087-70-1
- Ptelatoside B
Catalog No.:BCN4451
CAS No.:90852-99-6
- ent-Labda-8(17),13E-diene-3beta,15,18-triol
Catalog No.:BCN7662
CAS No.:90851-50-6
- Goshonoside F5
Catalog No.:BCN6442
CAS No.:90851-28-8
- 2-Benzoylpyridine
Catalog No.:BCC8562
CAS No.:91-02-1
- 2,6-Bis(hydroxymethyl)-p-cresol
Catalog No.:BCC8505
CAS No.:91-04-3
- Syringol
Catalog No.:BCN3534
CAS No.:91-10-1
- Coumarin
Catalog No.:BCN6309
CAS No.:91-64-5
- Benzoguanamine
Catalog No.:BCC8853
CAS No.:91-76-9
- N,N'-Bis(acetoacetyl)-o-toluidine
Catalog No.:BCC9062
CAS No.:91-96-3
- Fmoc-Arg-OH
Catalog No.:BCC3039
CAS No.:91000-69-0
- Impurity of Calcipotriol
Catalog No.:BCC5388
CAS No.:910133-69-6
- CGI-1746
Catalog No.:BCC1473
CAS No.:910232-84-7
- RGDS peptide
Catalog No.:BCC7694
CAS No.:91037-65-9
- BAY 60-6583
Catalog No.:BCC6197
CAS No.:910487-58-0
- Danshenol C
Catalog No.:BCN6681
CAS No.:910856-25-6
[Chemical constituents and antibacterial activity contained in Caesalpinia millettii].[Pubmed:23126193]
Zhongguo Zhong Yao Za Zhi. 2012 Jul;37(14):2105-7.
To study chemical constituents contained in roots of Caesalpinia millettii by HPLC. Six homoisoflavonoids were identified by spectroscopic data and physicochemical property as eucomin (1), intricatinol (2), 8-Methoxybonducellin (3), bonducellin (4), 8-methoxyisobonducellin (5) and 3-(4-methoxybenzyl) -5, 7-dimethoxychroman-4-one (6). All compounds were separated from the root of this genus for the first time. An antibacterial screening was made on eight monomeric compounds. Among them, 8-methoxyisobonducellin, intricatinol, bergenin, hyperoside and 11-O-galloylbergenin showed a inhibitory effect on Staphylococcus aureus, Klebsiella Peneumoniae, Beta streptococcus and Aeruginosus bacillus.
Flavonol galactoside caffeiate ester and homoisoflavones from Caesalpinia millettii HOOK. et ARN.[Pubmed:17409566]
Chem Pharm Bull (Tokyo). 2007 Apr;55(4):655-7.
Chemical examination of the stems of Caesalpinia millettii HOOK. et ARN. led to the isolation of new flavonol glycoside caffeiate ester (1) and homoisoflavone (2), along with four known homoisoflavones: eucomin (3), bonducellin (4), 8-Methoxybonducellin (5) and intricatinol (6). The structures of 1 and 2 were established to be tamarixetin 3-O-(6''-O-E-caffeoyl)-beta-D-galactopyranoside (1) and (Z)-7-hydroxy-8-methoxy-3-(4-methoxybenzyl) chroman-4-one (2) on the basis of detailed analyses of physical, chemical, and spectral data. Compounds 3-6 were isolated from this plant for the first time.
Synthesis, structural revision, and antioxidant activities of antimutagenic homoisoflavonoids from Hoffmanosseggia intricata.[Pubmed:17196384]
Bioorg Med Chem Lett. 2007 Mar 1;17(5):1288-90.
Intricatinol and intricatin, the two homoisoflavonoids isolated from Hoffmanosseggia intricata, and two analogs have been synthesized from pyrogallol in three steps. The spectral data of synthetic intricatinol are in good agreement with those of natural metabolite, but the spectral data of intricatin are not corroborative with those of the natural product. The structure of intricatin has been thus revised to 8-Methoxybonducellin, a compound isolated from Caesalpinia pulcherrima. The antioxidant activity of all the four homoisoflavonoids was determined by superoxide (NBT) and DPPH free radical scavenging methods. The synthetic analog 7,8-dihydroxy-3-[(3,4-dihydroxyphenyl)methylene]chroman-4-one displayed excellent activity in both methods.


