Calyxin BCAS# 164991-53-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 164991-53-1 | SDF | Download SDF |
| PubChem ID | 9829786 | Appearance | Yellow powder |
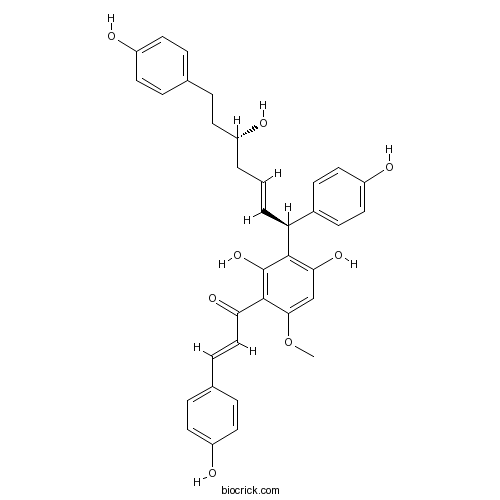
| Formula | C35H34O8 | M.Wt | 582.7 |
| Type of Compound | Chalcones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-1-[2,4-dihydroxy-3-[(E,1S,5S)-5-hydroxy-1,7-bis(4-hydroxyphenyl)hept-2-enyl]-6-methoxyphenyl]-3-(4-hydroxyphenyl)prop-2-en-1-one | ||
| SMILES | COC1=CC(=C(C(=C1C(=O)C=CC2=CC=C(C=C2)O)O)C(C=CCC(CCC3=CC=C(C=C3)O)O)C4=CC=C(C=C4)O)O | ||
| Standard InChIKey | DHYXVFFHVYUZJU-PEVIGJGQSA-N | ||
| Standard InChI | InChI=1S/C35H34O8/c1-43-32-21-31(41)33(35(42)34(32)30(40)20-10-23-8-16-27(38)17-9-23)29(24-11-18-28(39)19-12-24)4-2-3-25(36)13-5-22-6-14-26(37)15-7-22/h2,4,6-12,14-21,25,29,36-39,41-42H,3,5,13H2,1H3/b4-2+,20-10+/t25-,29+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Calyxin B exhibits potent activity against human HT-1080 fibrosarcoma cells with an ED50 value of 0.69 microM. 2. A methylated product of calyxin A and an epimeric mixture of Calyxin B, showed greatly reduced activity suggesting that phenolic hydroxyl groups are involved in the inhibitory activity. |
| Targets | NO |

Calyxin B Dilution Calculator

Calyxin B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7161 mL | 8.5807 mL | 17.1615 mL | 34.323 mL | 42.9037 mL |
| 5 mM | 0.3432 mL | 1.7161 mL | 3.4323 mL | 6.8646 mL | 8.5807 mL |
| 10 mM | 0.1716 mL | 0.8581 mL | 1.7161 mL | 3.4323 mL | 4.2904 mL |
| 50 mM | 0.0343 mL | 0.1716 mL | 0.3432 mL | 0.6865 mL | 0.8581 mL |
| 100 mM | 0.0172 mL | 0.0858 mL | 0.1716 mL | 0.3432 mL | 0.429 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- N9-Methylharman
Catalog No.:BCN3368
CAS No.:16498-64-9
- ML352
Catalog No.:BCC6434
CAS No.:1649450-12-3
- Azaperone
Catalog No.:BCC4630
CAS No.:1649-18-9
- G-5555
Catalog No.:BCC8095
CAS No.:1648863-90-4
- Podophyllotoxin 4-O-glucoside
Catalog No.:BCN8064
CAS No.:16481-54-2
- 4-(3,4-Dimethoxyphenyl)-3-butene-1,2-diol
Catalog No.:BCN1537
CAS No.:164661-12-5
- CGP60474
Catalog No.:BCC1474
CAS No.:164658-13-3
- Dutasteride
Catalog No.:BCC1097
CAS No.:164656-23-9
- Efinaconazole
Catalog No.:BCC5523
CAS No.:164650-44-6
- Aldicarb sulfone
Catalog No.:BCC5476
CAS No.:1646-88-4
- KPT-9274
Catalog No.:BCC6461
CAS No.:1643913-93-2
- Immepip dihydrobromide
Catalog No.:BCC6853
CAS No.:164391-47-3
- Kukoamine B
Catalog No.:BCN3836
CAS No.:164991-67-7
- Decumbenine B
Catalog No.:BCC8313
CAS No.:164991-68-8
- Orcinol gentiobioside
Catalog No.:BCN2815
CAS No.:164991-86-0
- Segetalin B
Catalog No.:BCC9247
CAS No.:164991-89-3
- Nα-Methylhistamine dihydrochloride
Catalog No.:BCC6701
CAS No.:16503-22-3
- Brevilin A
Catalog No.:BCN1727
CAS No.:16503-32-5
- 6-Epidemethylesquirolin D
Catalog No.:BCN1728
CAS No.:165074-00-0
- Shizukaol E
Catalog No.:BCN7880
CAS No.:165171-09-5
- Shizukaol G
Catalog No.:BCN7879
CAS No.:165171-11-9
- Cleroindicin E
Catalog No.:BCN1729
CAS No.:165197-71-7
- 1-Ethyl-3-nitrophthalate
Catalog No.:BCC8466
CAS No.:16533-45-2
- Hemiphroside A
Catalog No.:BCN1730
CAS No.:165338-27-2
Antiproliferative activity of diarylheptanoids from the seeds of Alpinia blepharocalyx.[Pubmed:11379774]
Biol Pharm Bull. 2001 May;24(5):525-8.
The 95% EtOH extract of the seeds of Alpinia blepharocalyx (Zingiberaceae) showed significant antiproliferative activity towards human HT-1080 fibrosarcoma and murine colon 26-L5 carcinoma cells. Chemical investigation of the extract led to the isolation of forty-four new (1-44) and one known (45) diarylheptanoids, eleven phenolic compounds (46-56) together with beta-sitosterol glucoside (57). Almost all the isolated compounds showed significant antiproliferative activity in a concentration-dependent manner. Among the compounds, epicalyxin F (17) exhibited the most potent activity against the proliferation of colon 26-L5 carcinoma cells with an ED50 value of 0.89 microM, while Calyxin B (2) exhibited the most potent activity against human HT-1080 fibrosarcoma cells with an ED50 value of 0.69 microM. Moreover, calyxins B (2) and K (11), epicalyxins F (17), I (20) and K (22), 6-hydroxycalyxin F (25), blepharoCalyxin B (27) and mixtures of 7 and epicalyxin G (18) and of calyxin J (10) and epicalyxin J (21) possessed more potent activity than a clinically used anticancer drug, 5-fluorouracil, towards HT-1080 fibrosarcoma cells. Analysis of the structure activity relationship suggested that the position of the attachment of a chalcone or a flavanone moiety does not affect the activity, although their presence in association causes a substantial enhancement of the antiproliferative activity. Moreover, the conjugated double bond of the chalcone moiety and the phenolic hydroxyl group potentiate the antiproliferative activity of the compounds.
Inhibitory effect of diarylheptanoids on nitric oxide production in activated murine macrophages.[Pubmed:9586575]
Biol Pharm Bull. 1998 Apr;21(4):371-4.
Thirteen novel diarylheptanoids bearing a chalcone or a flavanone moiety (1-13), a new curcumin derivative, 1,2-dihydrobis(de-O-methyl)curcumin (14), and two known flavonoids (15 and 16) isolated from the seeds of Alpinia blepharocalyx K. Schum. were tested for their inhibitory effects on nitric oxide (NO) production in lipopolysaccaride (LPS)-activated murine macrophages J774.1 in vitro. All the tested compounds inhibited NO production in a concentration-dependent manner (IC50=36-568 microM). Among the compounds examined, blepharoCalyxin B (13) was the most potent inhibitor of NO production (IC50=36 microM). Analysis of the structure activity relationship among these novel diarylheptanoids led to the conclusion that the position of attachment of a chalcone or a flavanone to a diarylheptanoid does not affect their inhibitory potency although their presence in association causes a substantial enhancement of the inhibitory activity. Moreover, a conjugated double bond in a chalcone moiety potentiated the inhibitory activity. On the other hand, hexamethoxydeoxycalyxin A (17) and pentamethoxyCalyxin B (18), a methylated product of calyxin A (1) and an epimeric mixture of Calyxin B, showed greatly reduced activity suggesting that phenolic hydroxyl groups are involved in the inhibitory activity.


