Kukoamine BCAS# 164991-67-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 164991-67-7 | SDF | Download SDF |
| PubChem ID | 10346914 | Appearance | Brownish crystals |
| Formula | C28H42N4O6 | M.Wt | 530.7 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in methan | ||
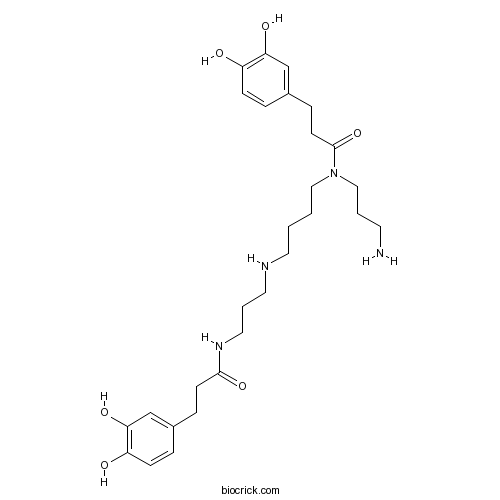
| Chemical Name | N-[3-[4-[3-aminopropyl-[3-(3,4-dihydroxyphenyl)propanoyl]amino]butylamino]propyl]-3-(3,4-dihydroxyphenyl)propanamide | ||
| SMILES | C1=CC(=C(C=C1CCC(=O)NCCCNCCCCN(CCCN)C(=O)CCC2=CC(=C(C=C2)O)O)O)O | ||
| Standard InChIKey | IWRAOCFRRTWUDF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C28H42N4O6/c29-13-3-18-32(28(38)12-8-22-6-10-24(34)26(36)20-22)17-2-1-14-30-15-4-16-31-27(37)11-7-21-5-9-23(33)25(35)19-21/h5-6,9-10,19-20,30,33-36H,1-4,7-8,11-18,29H2,(H,31,37) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Kukoamine B is a potent dual inhibitor for both Lipopolysaccharides (LPS) and oligodeoxynucleotides containing CpG motifs (CpG DNA), LPS and CpG DNA are important pathogenic molecules for the induction of sepsis,are drug targets for sepsis treatment, thus kukoamine B is worthy of further investigation as a potential candidate to treat sepsis. 2. Kukoamine B inhibits inflammation in septic mice by reducing the concentrations of plasma LPS, decreasing leukocyte sequestration and interfering with NFκB activation, and, therefore, suppressing the proadhesive phenotype of endothelial cells. 3. Kukoamine B has protective effects against hydrogen peroxide (H2O2) induced cell injury and potential mechanisms in SH-SY5Y cells, it may potentially serve as an agent for prevention of several human neurodegenerative and other disorders caused by oxidative stress. |
| Targets | PI3K | Akt | JNK | ERK | MMP(e.g.TIMP) | ROS | SOD | IL Receptor | NF-kB |

Kukoamine B Dilution Calculator

Kukoamine B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8843 mL | 9.4215 mL | 18.843 mL | 37.6861 mL | 47.1076 mL |
| 5 mM | 0.3769 mL | 1.8843 mL | 3.7686 mL | 7.5372 mL | 9.4215 mL |
| 10 mM | 0.1884 mL | 0.9422 mL | 1.8843 mL | 3.7686 mL | 4.7108 mL |
| 50 mM | 0.0377 mL | 0.1884 mL | 0.3769 mL | 0.7537 mL | 0.9422 mL |
| 100 mM | 0.0188 mL | 0.0942 mL | 0.1884 mL | 0.3769 mL | 0.4711 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Calyxin B
Catalog No.:BCN1726
CAS No.:164991-53-1
- N9-Methylharman
Catalog No.:BCN3368
CAS No.:16498-64-9
- ML352
Catalog No.:BCC6434
CAS No.:1649450-12-3
- Azaperone
Catalog No.:BCC4630
CAS No.:1649-18-9
- G-5555
Catalog No.:BCC8095
CAS No.:1648863-90-4
- Podophyllotoxin 4-O-glucoside
Catalog No.:BCN8064
CAS No.:16481-54-2
- 4-(3,4-Dimethoxyphenyl)-3-butene-1,2-diol
Catalog No.:BCN1537
CAS No.:164661-12-5
- CGP60474
Catalog No.:BCC1474
CAS No.:164658-13-3
- Dutasteride
Catalog No.:BCC1097
CAS No.:164656-23-9
- Efinaconazole
Catalog No.:BCC5523
CAS No.:164650-44-6
- Aldicarb sulfone
Catalog No.:BCC5476
CAS No.:1646-88-4
- KPT-9274
Catalog No.:BCC6461
CAS No.:1643913-93-2
- Decumbenine B
Catalog No.:BCC8313
CAS No.:164991-68-8
- Orcinol gentiobioside
Catalog No.:BCN2815
CAS No.:164991-86-0
- Segetalin B
Catalog No.:BCC9247
CAS No.:164991-89-3
- Nα-Methylhistamine dihydrochloride
Catalog No.:BCC6701
CAS No.:16503-22-3
- Brevilin A
Catalog No.:BCN1727
CAS No.:16503-32-5
- 6-Epidemethylesquirolin D
Catalog No.:BCN1728
CAS No.:165074-00-0
- Shizukaol E
Catalog No.:BCN7880
CAS No.:165171-09-5
- Shizukaol G
Catalog No.:BCN7879
CAS No.:165171-11-9
- Cleroindicin E
Catalog No.:BCN1729
CAS No.:165197-71-7
- 1-Ethyl-3-nitrophthalate
Catalog No.:BCC8466
CAS No.:16533-45-2
- Hemiphroside A
Catalog No.:BCN1730
CAS No.:165338-27-2
- Hemiphroside B
Catalog No.:BCN1731
CAS No.:165338-28-3
Neuroprotective effects of Kukoamine B against hydrogen peroxide-induced apoptosis and potential mechanisms in SH-SY5Y cells.[Pubmed:26164594]
Environ Toxicol Pharmacol. 2015 Jul;40(1):230-40.
Oxidative stress mediates the cell damage in several neurodegenerative diseases, including multiple sclerosis, Alzheimer's disease (AD) and Parkinson's disease (PD). This study aimed at investigating the protective effects of Kukoamine B (KuB) against hydrogen peroxide (H2O2) induced cell injury and potential mechanisms in SH-SY5Y cells. Our results revealed that treatment with KuB prior to H2O2 exposure effectively increased the cell viability, and restored the mitochondria membrane potential (MMP). Furthermore, KuB enhanced the antioxidant enzyme activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) and decreased the malondialdehyde (MDA) content. Moreover, KuB minimized the ROS formation and inhibited mitochondria-apoptotic pathway, MAPKs (p-p38, p-JNK, p-ERK) pathways, but activated PI3K-AKT pathway. In conclusion, we believed that KuB may potentially serve as an agent for prevention of several human neurodegenerative and other disorders caused by oxidative stress.
Kukoamine B, a novel dual inhibitor of LPS and CpG DNA, is a potential candidate for sepsis treatment.[Pubmed:21108626]
Br J Pharmacol. 2011 Mar;162(6):1274-90.
BACKGROUND AND PURPOSE: Lipopolysaccharides (LPS) and oligodeoxynucleotides containing CpG motifs (CpG DNA) are important pathogenic molecules for the induction of sepsis, and thus are drug targets for sepsis treatment. The present drugs for treating sepsis act only against either LPS or CpG DNA. Hence, they are not particularly efficient at combating sepsis as the latter two molecules usually cooperate during sepsis. In this study, a natural alkaloid compound Kukoamine B (KB) is presented as a potent dual inhibitor for both LPS and CpG DNA. EXPERIMENTAL APPROACH: The affinities of KB for LPS and CpG DNA were assessed using biosensor technology. Direct interaction of KB with LPS and CpG DNA were evaluated using neutralization assays. Selective inhibitory activities of KB on pro-inflammatory signal transduction and cytokine expression induced by LPS and CpG DNA were analysed by cellular assays. Protective effects of KB in a sepsis model in mice were elucidated by determining survival and circulatory LPS and tumour necrosis factor-alpha (TNF-alpha) concentrations. KEY RESULTS: KB had high affinities for LPS and CpG DNA. It neutralized LPS and CpG DNA and prevented them from interacting with mouse macrophages. KB selectively inhibited LPS- and CpG DNA-induced signal transduction and expression of pro-inflammatory mediators without interfering with signal pathways or cell viability in macrophages. KB protected mice challenged with heat-killed Escherichia coli, and reduced the circulatory levels of LPS and TNF-alpha. CONCLUSIONS AND IMPLICATIONS: This is the first report of a novel dual inhibitor of LPS and CpG DNA. KB is worthy of further investigation as a potential candidate to treat sepsis.


