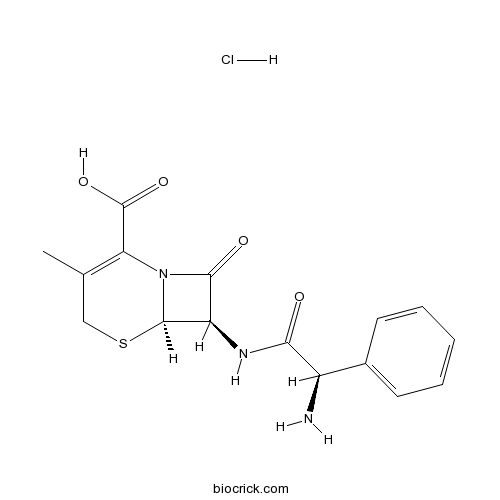
Cephalexin hydrochlorideCAS# 59695-59-9 |
- CYT387
Catalog No.:BCC2196
CAS No.:1056634-68-4
- Baricitinib phosphate
Catalog No.:BCC1401
CAS No.:1187595-84-1
- JAK2 Inhibitor V, Z3
Catalog No.:BCC1667
CAS No.:195371-52-9
- TG101209
Catalog No.:BCC2198
CAS No.:936091-14-4
- XL019
Catalog No.:BCC2057
CAS No.:945755-56-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 59695-59-9 | SDF | Download SDF |
| PubChem ID | 9951998 | Appearance | Powder |
| Formula | C16H18ClN3O4S | M.Wt | 383.85 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Cefalexin hydrochloride; Cephacillin hydrochloride | ||
| Solubility | Soluble in DMSO | ||
| Chemical Name | (6R,7R)-7-[[(2R)-2-amino-2-phenylacetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;hydrochloride | ||
| SMILES | CC1=C(N2C(C(C2=O)NC(=O)C(C3=CC=CC=C3)N)SC1)C(=O)O.Cl | ||
| Standard InChIKey | LSBUIZREQYVRSY-CYJZLJNKSA-N | ||
| Standard InChI | InChI=1S/C16H17N3O4S.ClH/c1-8-7-24-15-11(14(21)19(15)12(8)16(22)23)18-13(20)10(17)9-5-3-2-4-6-9;/h2-6,10-11,15H,7,17H2,1H3,(H,18,20)(H,22,23);1H/t10-,11-,15-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cefalexin hydrochloride is a cephalosporin antibiotic.
Target: Antibacterial
Cefalexin (INN, BAN) or cephalexin (USAN, AAN) is a first-generation cephalosporin antibiotic introduced in 1967 by Eli Lilly and Company. It is an orally administered agent with a similar antimicrobial spectrum to the intravenous agents cefalotin and cefazolin. It was first marketed as Keflex (Lilly), and is marketed under several other trade names. As of 2008, cefalexin was the most popular cephalosporin antibiotic in the United States, with more than 25 million prescriptions of its generic versions alone, for US$255 million in sales (though less popular than two other antibiotics, amoxicillin and azithromycin, each with 50 million prescriptions per year).
Cefalexin is marketed by generic pharmaceutical manufacturers under a wide range of brand names, including: Apo-Cephalex, Biocef, Cefanox, Ceforal, Cephabos, Cephalexin, Cephorum, Ceporex, Cilex, Ialex, Ibilex, Kefexin, Keflet, Keflex, Rekosporin, Keforal, Keftab, Keftal, Lopilexin, Larixin, Novo-Lexin, Ospexin, Tenkorex, Zephalexin, Panixine Disperdose, Rancef, Sialexin, Sporidex and Ulexin. A version of Keflex 750 mg capsules is marketed for twice-daily dosage, to improve compliance. However, it is not a sustained release formulation, and since it is more expensive than the older strengths, some physicians prescribe three 250 mg capsules to be taken twice daily, as a less expensive alternative. References: | |||||

Cephalexin hydrochloride Dilution Calculator

Cephalexin hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6052 mL | 13.0259 mL | 26.0518 mL | 52.1037 mL | 65.1296 mL |
| 5 mM | 0.521 mL | 2.6052 mL | 5.2104 mL | 10.4207 mL | 13.0259 mL |
| 10 mM | 0.2605 mL | 1.3026 mL | 2.6052 mL | 5.2104 mL | 6.513 mL |
| 50 mM | 0.0521 mL | 0.2605 mL | 0.521 mL | 1.0421 mL | 1.3026 mL |
| 100 mM | 0.0261 mL | 0.1303 mL | 0.2605 mL | 0.521 mL | 0.6513 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Cefalexin hydrochloride is a cephalosporin antibiotic.
- 6alpha-Hydroxyhispanone
Catalog No.:BCN7416
CAS No.:596814-48-1
- 3-O-Acetyl-beta-boswellic acid
Catalog No.:BCN2672
CAS No.:5968-70-7
- Calyciphylline A
Catalog No.:BCN4098
CAS No.:596799-30-3
- AC 55649
Catalog No.:BCC7359
CAS No.:59662-49-6
- Alpha-Obscurine
Catalog No.:BCN6701
CAS No.:596-55-4
- Glycopyrrolate
Catalog No.:BCC4275
CAS No.:596-51-0
- H-D-Gln-OH
Catalog No.:BCC2920
CAS No.:5959-95-5
- 3-Amino-2-naphthoic acid
Catalog No.:BCC8607
CAS No.:5959-52-4
- Carnosol
Catalog No.:BCN1055
CAS No.:5957-80-2
- H-D-Ala-OtBu.HCl
Catalog No.:BCC2849
CAS No.:59531-86-1
- Boc-Ser-OBzl
Catalog No.:BCC3440
CAS No.:59524-02-6
- Megestrol Acetate
Catalog No.:BCC4365
CAS No.:595-33-5
- Piperacillin Sodium
Catalog No.:BCC4704
CAS No.:59703-84-3
- Camostat Mesilate
Catalog No.:BCC4894
CAS No.:59721-29-8
- Citalopram hydrobromide
Catalog No.:BCC7063
CAS No.:59729-32-7
- beta-Amyrin palmitate
Catalog No.:BCN4099
CAS No.:5973-06-8
- 1beta,10beta-Epoxy-6beta-isobutyryloxy-9-oxofuranoeremophilane
Catalog No.:BCN7601
CAS No.:59742-11-9
- Cudraflavanone B
Catalog No.:BCN3446
CAS No.:597542-74-0
- Boc-Asp(OMe)-OH.DCHA
Catalog No.:BCC3367
CAS No.:59768-74-0
- 6beta-Angeloyloxy-1beta,10beta-epoxy-9-oxofuranoeremophilane
Catalog No.:BCN7600
CAS No.:59780-08-4
- Cyclosporin C
Catalog No.:BCC8160
CAS No.:59787-61-0
- Vindolinine
Catalog No.:BCN7822
CAS No.:5980-02-9
- UK 14,304
Catalog No.:BCC5226
CAS No.:59803-98-4
- Tenoxicam
Catalog No.:BCC4733
CAS No.:59804-37-4
Comparative study of cephalexin hydrochloride and cephalexin monohydrate in the treatment of skin and soft tissue infections.[Pubmed:3046484]
Antimicrob Agents Chemother. 1988 Jun;32(6):882-5.
In two prospective, randomized multicenter double-blind studies with a dosage of either 250 mg given four times a day (study A) or 500 mg given two times a day (study B), the comparative efficacy and safety of Cephalexin hydrochloride (LY061188; Keftab) and cephalexin monohydrate (Keflex) for treatment of skin and soft tissue infections were determined. In study A, 97 patients received Cephalexin hydrochloride and 101 patients received cephalexin monohydrate. In study B, 75 patients received Cephalexin hydrochloride and 70 patients received cephalexin monohydrate. Diagnoses included abscesses, cellulitis, wound infections, and infected dermatitis, and were comparable in the different treatment groups. Pathogens were isolated from 82% of patients enrolled; the majority of isolates were of Staphylococcus aureus, Streptococcus pyogenes, other staphylococcal species, and a few gram-negative bacteria. In study A, 68 of 71 (95.7%) evaluable patients who received Cephalexin hydrochloride responded satisfactorily; 73 of 81 (90%) patients who received cephalexin monohydrate also responded satisfactorily. In study B, 56 of 58 (96.5%) evaluable patients who received Cephalexin hydrochloride responded satisfactorily; 47 of 50 (94%) patients who received cephalexin monohydrate also responded satisfactorily. An adverse clinical event leading to discontinuation of the treatment drug developed in 17 of 343 (4.95%) patients in both studies. No differences were noted between the two drugs. Skin eruptions, pruritus, and mild gastrointestinal symptoms were the common adverse effects. These data suggest that Cephalexin hydrochloride, a new formulation of cephalexin, is a safe and effective antimicrobial agent for treatment of a variety of skin and subcutaneous infections in a dosage of either 250 mg four times a day or 500 mg twice a day.


