Compound 401Selective DNA-PK and mTOR inhibitor CAS# 168425-64-7 |
- Fosbretabulin (Combretastatin A4 Phosphate (CA4P)) Disodium
Catalog No.:BCC4600
CAS No.:168555-66-6
- Eribulin
Catalog No.:BCC5174
CAS No.:253128-41-5
- Eribulin mesylate
Catalog No.:BCC5173
CAS No.:441045-17-6
- CYT997 (Lexibulin)
Catalog No.:BCC4601
CAS No.:917111-44-5
- MPC 6827 hydrochloride
Catalog No.:BCC8040
CAS No.:917369-31-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 168425-64-7 | SDF | Download SDF |
| PubChem ID | 10039361 | Appearance | Powder |
| Formula | C16H15N3O2 | M.Wt | 281.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 6 mg/mL (21.33 mM; Need ultrasonic and warming) | ||
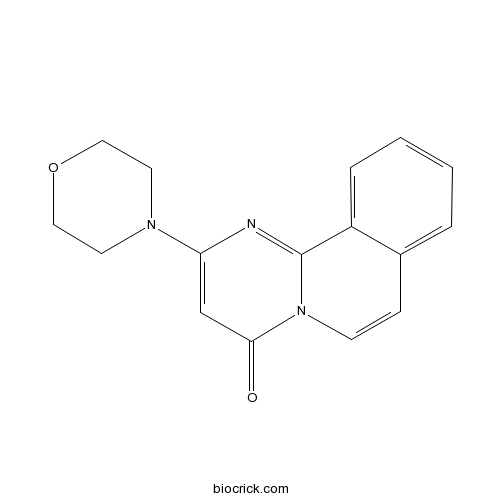
| Chemical Name | 2-morpholin-4-ylpyrimido[2,1-a]isoquinolin-4-one | ||
| SMILES | C1COCCN1C2=CC(=O)N3C=CC4=CC=CC=C4C3=N2 | ||
| Standard InChIKey | BVRDQVRQVGRNHG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H15N3O2/c20-15-11-14(18-7-9-21-10-8-18)17-16-13-4-2-1-3-12(13)5-6-19(15)16/h1-6,11H,7-10H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Reversible and selective inhibitor of DNA-dependent protein kinase (DNA-PK) and mammalian target of rapamycin (mTOR) (IC50 values are 0.28 and 5.3 μM respectively). Displays little affinity for other commonly studied kinases including PI 3-K, ATM and ATR (IC50 values are all > 100 μM). Induces apoptosis in vitro. |

Compound 401 Dilution Calculator

Compound 401 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5548 mL | 17.774 mL | 35.548 mL | 71.0959 mL | 88.8699 mL |
| 5 mM | 0.711 mL | 3.5548 mL | 7.1096 mL | 14.2192 mL | 17.774 mL |
| 10 mM | 0.3555 mL | 1.7774 mL | 3.5548 mL | 7.1096 mL | 8.887 mL |
| 50 mM | 0.0711 mL | 0.3555 mL | 0.711 mL | 1.4219 mL | 1.7774 mL |
| 100 mM | 0.0355 mL | 0.1777 mL | 0.3555 mL | 0.711 mL | 0.8887 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Compound 401 is a synthetic inhibitor of DNA-PK (IC50 = 0.28 μM) that also targets mTOR but not PI3K in vitro.
In Vitro:Compound 401 is a potent inhibitor of DNA-PK (IC50=0.28 μM). Compound 401 is reported to be a poor inhibitor of PI3K, ATM, and ATR in vitro, but it is active against mTOR. Compound 401 shows activity against mTOR (IC50=5.3 μM) but not p110α/p85α PI3K (IC50>100 μM). Treatment of cells with Compound 401 blocks the phosphorylation of sites modified by mTOR-Raptor and mTOR-Rictor complexes (ribosomal protein S6 kinase 1 Thr389 and Akt Ser473, respectively). By contrast, there is no direct inhibition of Akt Thr308 phosphorylation, which is dependent on PI3K. Similar effects are also observed in cells that lack DNA-PK. Compound 401 inhibits immunoprecipitated epitope-tagged mTOR or endogenous mTOR in Raptor immunoprecipitates. In both cases, inhibition of 67% or 78% is obtained at 5 μM or 10 μM Compound 401, respectively. By contrast, dose response curves show that the p110α/p85α or p110β/p85α PI3K complexes are poorly inhibited by Compound 401 at these concentrations. The proliferation of TSC1-/- fibroblasts is inhibited in the presence of Compound 401, but TSC1+/+ cells are resistant[1].
References:
[1]. Ballou LM, et al. Inhibition of mammalian target of rapamycin signaling by 2-(morpholin-1-yl)pyrimido[2,1-alpha]isoquinolin-4-one. J Biol Chem. 2007 Aug 17;282(33):24463-70.
- Tacrine hydrochloride
Catalog No.:BCC6869
CAS No.:1684-40-8
- Oxymatrine
Catalog No.:BCN1103
CAS No.:16837-52-8
- Asiaticoside
Catalog No.:BCN1011
CAS No.:16830-15-2
- 3-Epidehydropachymic acid
Catalog No.:BCN3644
CAS No.:168293-15-0
- 3-O-Acetyl-16 alpha-hydroxydehydrotrametenolic acid
Catalog No.:BCN1531
CAS No.:168293-14-9
- 3-O-Acetyl-16 alpha-hydroxytrametenolic acid
Catalog No.:BCN1532
CAS No.:168293-13-8
- C-Veratroylglycol
Catalog No.:BCN1102
CAS No.:168293-10-5
- 3,5-Dimethoxy-3'-hydroxybibenzyl
Catalog No.:BCN8112
CAS No.:168281-05-8
- Rimonabant
Catalog No.:BCC4414
CAS No.:168273-06-1
- Evofolin B
Catalog No.:BCN1101
CAS No.:168254-96-4
- Wilforol C
Catalog No.:BCN1100
CAS No.:168254-95-3
- Nortenuazonic acid
Catalog No.:BCN1847
CAS No.:16820-44-3
- Rhodiocyanoside A
Catalog No.:BCN7852
CAS No.:168433-86-1
- Epifriedelanol
Catalog No.:BCN1104
CAS No.:16844-71-6
- SHU 9119
Catalog No.:BCC6019
CAS No.:168482-23-3
- Z-D-Val-OH
Catalog No.:BCC2732
CAS No.:1685-33-2
- Dehydrogeijerin
Catalog No.:BCN7531
CAS No.:16850-91-2
- Fosbretabulin (Combretastatin A4 Phosphate (CA4P)) Disodium
Catalog No.:BCC4600
CAS No.:168555-66-6
- H-Asp(OMe)-OH.HCl
Catalog No.:BCC2889
CAS No.:16856-13-6
- AIDA
Catalog No.:BCC6841
CAS No.:168560-79-0
- TPEN
Catalog No.:BCC7913
CAS No.:16858-02-9
- Conivaptan HCl
Catalog No.:BCC3756
CAS No.:168626-94-6
- Ezatiostat
Catalog No.:BCC3638
CAS No.:168682-53-9
- H-Tyr-OtBu
Catalog No.:BCC3128
CAS No.:16874-12-7
The oncolytic compound LTX-401 targets the Golgi apparatus.[Pubmed:27588704]
Cell Death Differ. 2016 Dec;23(12):2031-2041.
LTX-401 is an oncolytic amino acid derivative with potential immunogenic properties. Here, we demonstrate that LTX-401 selectively destroys the structure of the Golgi apparatus, as determined by means of ultrastructural analyses and fluorescence microscopic observation of cells expressing Golgi-targeted GFP reporters. Subcellular fractionation followed by mass spectrometric detection revealed that LTX-401 selectively enriched in the Golgi rather than in mitochondria or in the cytosol. The Golgi-dissociating agent Brefeldin A (BFA) reduced cell killing by LTX-401 as it partially inhibited LTX-401-induced mitochondrial release of cytochrome c and the activation of BAX. The cytotoxic effect of LTX-401 was attenuated by the double knockout of BAX and BAK, as well as the mitophagy-enforced depletion of mitochondria, yet was refractory to caspase inhibition. LTX-401 induced all major hallmarks of immunogenic cell death detectable with biosensor cell lines including calreticulin exposure, ATP release, HMGB1 exodus and a type-1 interferon response. Moreover, LTX-401-treated tumors manifested a strong lymphoid infiltration. Altogether these results support the contention that LTX-401 can stimulate immunogenic cell death through a pathway in which Golgi-localized LTX-401 operates upstream of mitochondrial membrane permeabilization.
Inhibition of histamine release induced by compound 48/80 and peptide 401 in the presence and absence of calcium. Implications for the mode of action of anti-allergic compounds.[Pubmed:6157320]
Agents Actions. 1980 Jun;10(3):222-8.
Histamine may be released from rat peritoneal mast cells by compound 48/80 and peptide 401 in the presence and absence of extracellular calcium. The process is non-cytolytic and requires an intact cell metabolism. The release produced under both conditions is inhibited by disodium cromoglycate, theophylline, dibutyryl cyclic AMP and (at high concentrations) quercetin. The efficacy of the drugs in the absence of extracellular calcium cannot be explained in terms of their postulated effect on the calcium-gating mechanism operative in anaphylactic secretion. Alternative modes of action of the compounds are thus considered.
Anti-inflammatory activity of bee venom peptide 401 (mast cell degranulating peptide) and compound 48/80 results from mast cell degranulation in vivo.[Pubmed:2328399]
Br J Pharmacol. 1990 Feb;99(2):350-4.
1. The relationship between the anti-inflammatory activity of the bee venom peptide 401 in the carrageenin-induced oedema of the rat hind paw and its mast cell degranulating activity has been reinvestigated. 2. Mast cell degranulation caused by compound 48/80 (10 mg kg-1) or by allergen challenge in rats sensitized to Nippostrongylus brasiliensis also suppressed rat hind paw oedema in the same test. 3. The anti-inflammatory activities of peptide 401 and compound 48/80 were partially suppressed by pretreatment of rats with mepyramine and methysergide, at doses (2.5 mg kg-1) that completely suppressed skin reactions to these mast cell-derived amines. Pretreatment of rats with compound 48/80 also suppressed the apparent anti-inflammatory actions of peptide 401 and of compound 48/80. 4. Injection of peptide 401 together with carrageenin increased the inflammatory response in the rat hind paw. 5. The anti-inflammatory activity of peptide 401 and of compound 48/80 in the carrageenin-induced swelling of the rat hind paw arises from mast cell degranulation in vivo.
Inhibition of mammalian target of rapamycin signaling by 2-(morpholin-1-yl)pyrimido[2,1-alpha]isoquinolin-4-one.[Pubmed:17562705]
J Biol Chem. 2007 Aug 17;282(33):24463-70.
Signaling through the mammalian target of rapamycin (mTOR) is hyperactivated in many human tumors, including hamartomas associated with tuberous sclerosis complex (TSC). Several small molecules such as LY294002 inhibit mTOR kinase activity, but they also inhibit phosphatidylinositol 3-kinase (PI3K) at similar concentrations. Compound 401 is a synthetic inhibitor of DNA-dependent protein kinase (DNA-PK) that also targets mTOR but not PI3K in vitro (Griffin, R. J., Fontana, G., Golding, B. T., Guiard, S., Hardcastle, I. R., Leahy, J. J., Martin, N., Richardson, C., Rigoreau, L., Stockley, M., and Smith, G. C. (2005) J. Med. Chem. 48, 569-585). We used 401 to test the cellular effect of mTOR inhibition without the complicating side effects on PI3K. Treatment of cells with 401 blocked the phosphorylation of sites modified by mTOR-Raptor and mTOR-Rictor complexes (ribosomal protein S6 kinase 1 Thr(389) and Akt Ser(473), respectively). By contrast, there was no direct inhibition of Akt Thr(308) phosphorylation, which is dependent on PI3K. Similar effects were also observed in cells that lack DNA-PK. The proliferation of TSC1-/- fibroblasts was inhibited in the presence of 401, but TSC1+/+ cells were resistant. In contrast to rapamycin, long-term treatment of TSC1-/- cells with 401 did not up-regulate phospho-Akt Ser(473). Because increased Akt activity promotes survival, this may explain why the level of apoptosis was increased in the presence of 401 but not rapamycin. These results suggest that mTOR kinase inhibitors might be more effective than rapamycins in controlling the growth of TSC hamartomas and other tumors that depend on elevated mTOR activity.
Selective benzopyranone and pyrimido[2,1-a]isoquinolin-4-one inhibitors of DNA-dependent protein kinase: synthesis, structure-activity studies, and radiosensitization of a human tumor cell line in vitro.[Pubmed:15658870]
J Med Chem. 2005 Jan 27;48(2):569-85.
A diverse range of chromen-2-one, chromen-4-one and pyrimidoisoquinolin-4-one derivatives was synthesized and evaluated for inhibitory activity against the DNA repair enzyme DNA-dependent protein kinase (DNA-PK), with a view to elucidating structure-activity relationships for potency and kinase selectivity. DNA-PK inhibitory activity varied widely over the series of compounds evaluated (IC(50) values ranged from 0.19 to >10 microM), with excellent activity being observed for the 7,8-benzochromen-4-one and pyrimido[2,1-a]isoquinolin-4-one templates. By contrast, inhibitors based on the benzochromen-2-one (coumarin) or 2-aryl-7,8-benzochromen-4-one (flavone) scaffolds were less potent. Crucially, these studies revealed a very constrained structure-activity relationship at the 2-position of the benzopyranone and pyrimido[2,1-a]isoquinolin-4-one pharmacophore, with only a 2-morpholino or 2-(2'-methylmorpholino) group being tolerated at this position. More detailed biological studies conducted with the most potent inhibitor NU7163 (48; IC(50) = 0.19 microM) demonstrated ATP-competitive DNA-PK inhibition, with a K(i) value of 24 nM, and 48 exhibited selectivity for DNA-PK compared with the related enzymes ATM, ATR, mTOR, and PI 3-K (p110alpha). Compound 48 sensitized the HeLa human tumor cell line to the cytotoxic effects of ionizing radiation in vitro, a dose modification factor of 2.3 at 10% survival being observed with an inhibitor concentration of 5 microM. This study identified these structural classes as novel DNA-PK inhibitors and delineated initial structure-activity relationships against DNA-PK.


