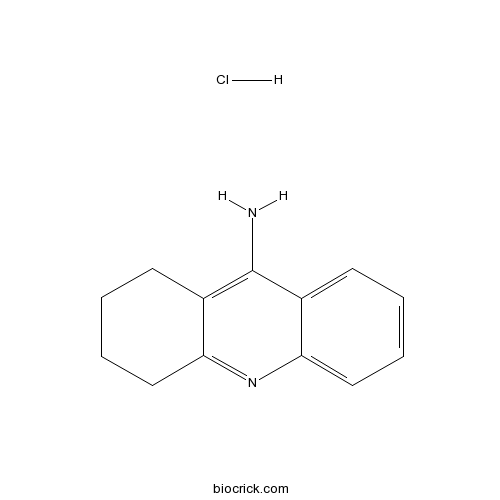
Tacrine hydrochlorideCholinesterase inhibitor CAS# 1684-40-8 |
- (24R)-MC 976
Catalog No.:BCC1289
CAS No.:112828-09-8
- (24S)-MC 976
Catalog No.:BCC1291
CAS No.:112849-14-6
- 1alpha, 25-Dihydroxy VD2-D6
Catalog No.:BCC1299
CAS No.:216244-04-1
- 1alpha, 24, 25-Trihydroxy VD2
Catalog No.:BCC1298
CAS No.:457048-34-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1684-40-8 | SDF | Download SDF |
| PubChem ID | 2723754 | Appearance | Powder |
| Formula | C13H15ClN2 | M.Wt | 234.73 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
| Chemical Name | 1,2,3,4-tetrahydroacridin-9-amine;hydrochloride | ||
| SMILES | C1CCC2=NC3=CC=CC=C3C(=C2C1)N.Cl | ||
| Standard InChIKey | ZUFVXZVXEJHHBN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H14N2.ClH/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13;/h1,3,5,7H,2,4,6,8H2,(H2,14,15);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent cholinesterase inhibitor, a cognition enhancer in vivo. |

Tacrine hydrochloride Dilution Calculator

Tacrine hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2602 mL | 21.3011 mL | 42.6021 mL | 85.2043 mL | 106.5053 mL |
| 5 mM | 0.852 mL | 4.2602 mL | 8.5204 mL | 17.0409 mL | 21.3011 mL |
| 10 mM | 0.426 mL | 2.1301 mL | 4.2602 mL | 8.5204 mL | 10.6505 mL |
| 50 mM | 0.0852 mL | 0.426 mL | 0.852 mL | 1.7041 mL | 2.1301 mL |
| 100 mM | 0.0426 mL | 0.213 mL | 0.426 mL | 0.852 mL | 1.0651 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Oxymatrine
Catalog No.:BCN1103
CAS No.:16837-52-8
- Asiaticoside
Catalog No.:BCN1011
CAS No.:16830-15-2
- 3-Epidehydropachymic acid
Catalog No.:BCN3644
CAS No.:168293-15-0
- 3-O-Acetyl-16 alpha-hydroxydehydrotrametenolic acid
Catalog No.:BCN1531
CAS No.:168293-14-9
- 3-O-Acetyl-16 alpha-hydroxytrametenolic acid
Catalog No.:BCN1532
CAS No.:168293-13-8
- C-Veratroylglycol
Catalog No.:BCN1102
CAS No.:168293-10-5
- 3,5-Dimethoxy-3'-hydroxybibenzyl
Catalog No.:BCN8112
CAS No.:168281-05-8
- Rimonabant
Catalog No.:BCC4414
CAS No.:168273-06-1
- Evofolin B
Catalog No.:BCN1101
CAS No.:168254-96-4
- Wilforol C
Catalog No.:BCN1100
CAS No.:168254-95-3
- Nortenuazonic acid
Catalog No.:BCN1847
CAS No.:16820-44-3
- Agitoxin 2
Catalog No.:BCC8026
CAS No.:168147-41-9
- Compound 401
Catalog No.:BCC7622
CAS No.:168425-64-7
- Rhodiocyanoside A
Catalog No.:BCN7852
CAS No.:168433-86-1
- Epifriedelanol
Catalog No.:BCN1104
CAS No.:16844-71-6
- SHU 9119
Catalog No.:BCC6019
CAS No.:168482-23-3
- Z-D-Val-OH
Catalog No.:BCC2732
CAS No.:1685-33-2
- Dehydrogeijerin
Catalog No.:BCN7531
CAS No.:16850-91-2
- Fosbretabulin (Combretastatin A4 Phosphate (CA4P)) Disodium
Catalog No.:BCC4600
CAS No.:168555-66-6
- H-Asp(OMe)-OH.HCl
Catalog No.:BCC2889
CAS No.:16856-13-6
- AIDA
Catalog No.:BCC6841
CAS No.:168560-79-0
- TPEN
Catalog No.:BCC7913
CAS No.:16858-02-9
- Conivaptan HCl
Catalog No.:BCC3756
CAS No.:168626-94-6
- Ezatiostat
Catalog No.:BCC3638
CAS No.:168682-53-9
Multifunctional liposomes for nasal delivery of the anti-Alzheimer drug tacrine hydrochloride.[Pubmed:24807822]
J Liposome Res. 2014 Dec;24(4):323-35.
The purpose of this study was the development of multifunctional liposomes for nasal administration of Tacrine hydrochloride. Liposomes were prepared using traditional excipients (cholesterol and phosphatidylcholine), partly enriched with alpha-tocopherol and/or Omega3 fatty acids. This approach was chosen in order to obtain at the same time two positive results: an enhanced drug permeation through nasal mucosa and a concomitant neuroprotective effect. Several liposome formulations were prepared using the Reverse Phase Evaporation technique followed by membrane filter extrusion. In particular, liposome capacity to enhance drug permeation was evaluated by means of membrane permeation and cellular uptake studies. Furthermore, liposome effect on neuronal viability and intracellular ROS production was evaluated as well as their cytoprotective effect against oxidative stress. All liposome formulations showed a mean diameter in the range of 175 nm to 219 nm with polydispersity index lower than 0.22, a lightly negative zeta potential and excellent encapsulation efficiency. Moreover, along with good mucoadhesive properties, multifunctional liposomes showed a markedly increase in tacrine permeability, which can be related to liposome fusion with cellular membrane, a hypothesis, which was also supported by cellular uptake studies. Finally, the addition of alpha-tocopherol without Omega3 fatty acids, was found to increase the neuroprotective activity and antioxidant properties of liposomes.
Transdermal iontophoretic delivery of tacrine hydrochloride: Correlation between in vitro permeation and in vivo performance in rats.[Pubmed:27633278]
Int J Pharm. 2016 Nov 20;513(1-2):393-403.
The aim of present investigation is to evaluate the feasibility of transdermal iontophoretic delivery of Tacrine hydrochloride in Sprague Dawley (SD) rats using anodal iontophoretic patches and to correlate plasma tacrine concentration profiles to in vitro tacrine permeation flux. In vitro skin permeation studies were carried out across artificial membrane CELGRAD((R)) 2400, freshly excised SD rat abdominal skin, freshly excised hairless rat abdominal skin, and frozen pig skin to examine the role of permeation membranes. Furthermore, plasma profiles with an application of 0.1-0.3mA current strength and tacrine concentration loading of 5-20mg/ml were obtained in SD rats. The tacrine plasma profiles were fitted to one-compartmental model using WinNonlin and in vivo transdermal absorption rates were then correlated to in vitro permeation profiles using various approaches. Tacrine permeation across membranes revealed current dependent interspecies differences at lower current strength application which diminished at higher current strength application, whereas, no significant difference in tacrine permeation was observed across fresh and frozen SD rat skin under 0.2mA current application. In vivo studies confirmed current and concentration dependent tacrine plasma profiles with possible tacrine depot formation under the skin in-line with earlier in vitro results. Correlation of in vivo transdermal absorption rates to in vitro permeation profiles revealed higher in vitro permeation fluxes compare to in vivo transdermal absorption rates at varied combination of current strength and concentrations. Present in vivo studies support the earlier published in vitro findings and tacrine plasma profiles show a potential to reach therapeutic effective concentration of Tacrine hydrochloride to provide a platform for pre-programmed tacrine delivery.
Application of design of experiments for formulation development and mechanistic evaluation of iontophoretic tacrine hydrochloride delivery.[Pubmed:27100474]
Drug Dev Ind Pharm. 2016 Nov;42(11):1894-902.
OBJECTIVE: The objective of this investigation is to develop mathematical equation to understand the impact of variables and establish statistical control over transdermal iontophoretic delivery of Tacrine hydrochloride. In addition, possibility of using conductivity measurements as a tool of predicting ionic mobility of the participating ions for the application of iontophoretic delivery was explored. METHODS: Central composite design was applied to study effect of independent variables like current strength, buffer molarity, and drug concentration on iontophoretic tacrine permeation flux. Molar conductivity was determined to evaluate electro-migration of tacrine ions with application of Kohlrausch's law. RESULTS: The developed mathematic equation not only reveals drug concentration as the most significant variable regulating tacrine permeation, followed by current strength and buffer molarity, but also is capable to optimize tacrine permeation with respective combination of independent variables to achieve desired therapeutic plasma concentration of tacrine in treatment of Alzheimer's disease. Moreover, relative higher mobility of sodium and chloride ions was observed as compared to estimated tacrine ion mobility. CONCLUSIONS: This investigation utilizes the design of experiment approach and extends the primary understanding of imapct of electronic and formulation variables on the tacrine permeation for the formulation development of iontophoretic tacrine delivery.
The orthorhombic pseudopolymorph of tacrine hydrochloride.[Pubmed:27698319]
Acta Crystallogr B Struct Sci Cryst Eng Mater. 2016 Oct 1;72(Pt 5):771-774.
Crystallization of Tacrine hydrochloride, an acetylcholinesterase inhibitor used during treatment of mild to moderate Alzheimer's disease, from a water:ethanol solution has resulted in an orthorhombic pseudopolymorph. This orthorhombic form which occurs as a dihydrate shows columns of stacking acridines together with continuous Cl-Owater-Owater-Cl chains and ladder-like ribbons composed of squares and hexagons.
A comparative study in rats of the in vitro and in vivo pharmacology of the acetylcholinesterase inhibitors tacrine, donepezil and NXX-066.[Pubmed:10193909]
Neuropharmacology. 1999 Jan;38(1):181-93.
The in vitro and in vivo effects of the novel acetylcholinesterase inhibitors donepezil and NXX-066 have been compared to tacrine. Using purified acetylcholinesterase from electric eel both tacrine and donepezil were shown to be reversible mixed type inhibitors, binding to a similar site on the enzyme. In contrast, NXX-066 was an irreversible non-competitive inhibitor. All three compounds were potent inhibitors of rat brain acetylcholinesterase (IC50 [nM]; tacrine: 125 +/- 23; NXX-066: 148 +/- 15; donepezil: 33 +/- 12). Tacrine was also a potent butyrylcholinesterase inhibitor. Donepezil and tacrine displaced [3H]pirenzepine binding in rat brain homogenates (IC50 values [microM]; tacrine: 0.7; donepezil: 0.5) but NXX-066 was around 80 times less potent at this M1-muscarinic site. Studies of carbachol stimulated increases in [Ca2+]i in neuroblastoma cells demonstrated that both donepezil and tacrine were M1 antagonists. Ligand binding suggested little activity of likely pharmacological significance with any of the drugs at other neurotransmitter sites. Intraperitoneal administration of the compounds to rats produced dose dependent increases in salivation and tremor (ED50 [micromol/kg]; tacrine: 15, NXX-066: 35, donepezil: 6) with NXX-066 having the most sustained effect on tremor. Following oral administration, NXX-066 had the slowest onset but the greatest duration of action. The relative potency also changed, tacrine having low potency (ED50 [micromol/kg]; tacrine: 200, NXX-066: 30, donepezil: 50). Salivation was severe only in tacrine treated animals. Using in vivo microdialysis in cerebral cortex, both NXX-066 and tacrine were found to produce a marked (at least 30-fold) increase in extracellular acetylcholine which remained elevated for more than 2 h after tacrine and 4 h after NXX-066.
Tetrahydro-9-aminoacridine has mixed actions on muscarinic currents and blocks opioid currents in rat locus ceruleus neurons.[Pubmed:8558423]
J Pharmacol Exp Ther. 1996 Jan;276(1):137-42.
Actions of tetrahydro-9-aminoacridine (THA) on membrane properties of locus ceruleus neurons were examined using intracellular recording in superfused brain slices. Low concentrations of THA (300 nM-3 microM) caused a small inward current and a 10-fold increase in the potency of ACh to produce inward (excitatory) currents. No effect was seen on currents activated by carbachol, a muscarinic agonist not degraded by cholinesterases. High concentrations of THA (30-300 microM) caused larger inward currents and a decrease in cell conductance. At these concentrations THA inhibited inward currents induced by carbachol (IC50 = 33 microM) and by substance P, which reportedly excites locus ceruleus neurons via the same ionic mechanism as muscarinic agonists. Furthermore, outward currents activated by opioids could be completely blocked (IC50 = 15 microM). Also affected was the action potential waveform, which was slower to rise, longer in duration and smaller in amplitude. The results suggest that THA has predominantly excitatory effects on locus ceruleus neurons--both by greatly enhancing the actions of ACh and by producing a small inward current. At high concentrations effects are mixed and include inhibition of muscarinic currents, as well as of resting and agonist-induced inwardly rectifying potassium currents. The block of opioid currents by THA was not consistent with inhibition of a cationic conductance as recently proposed.


