Ezatiostat(GST) P1-1 inhibitor CAS# 168682-53-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 168682-53-9 | SDF | Download SDF |
| PubChem ID | 5310939 | Appearance | Powder |
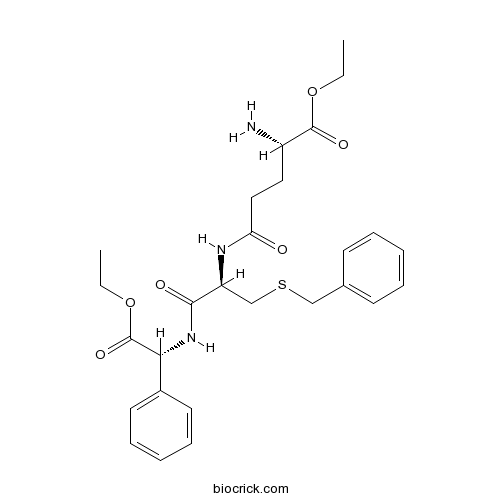
| Formula | C27H35N3O6S | M.Wt | 529.65 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | TER199;TLK199;Telintra | ||
| Solubility | DMSO : ≥ 100 mg/mL (188.80 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | ethyl (2S)-2-amino-5-[[(2R)-3-benzylsulfanyl-1-[[(1R)-2-ethoxy-2-oxo-1-phenylethyl]amino]-1-oxopropan-2-yl]amino]-5-oxopentanoate | ||
| SMILES | CCOC(=O)C(CCC(=O)NC(CSCC1=CC=CC=C1)C(=O)NC(C2=CC=CC=C2)C(=O)OCC)N | ||
| Standard InChIKey | GWEJFLVSOGNLSS-WPFOTENUSA-N | ||
| Standard InChI | InChI=1S/C27H35N3O6S/c1-3-35-26(33)21(28)15-16-23(31)29-22(18-37-17-19-11-7-5-8-12-19)25(32)30-24(27(34)36-4-2)20-13-9-6-10-14-20/h5-14,21-22,24H,3-4,15-18,28H2,1-2H3,(H,29,31)(H,30,32)/t21-,22-,24+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ezatiostat(TER199; TLK199) is a glutathione analog inhibitor of glutathione S-transferase (GST) P1-1. | |||||
| Targets | Gutathione S-transferase | |||||

Ezatiostat Dilution Calculator

Ezatiostat Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.888 mL | 9.4402 mL | 18.8804 mL | 37.7608 mL | 47.201 mL |
| 5 mM | 0.3776 mL | 1.888 mL | 3.7761 mL | 7.5522 mL | 9.4402 mL |
| 10 mM | 0.1888 mL | 0.944 mL | 1.888 mL | 3.7761 mL | 4.7201 mL |
| 50 mM | 0.0378 mL | 0.1888 mL | 0.3776 mL | 0.7552 mL | 0.944 mL |
| 100 mM | 0.0189 mL | 0.0944 mL | 0.1888 mL | 0.3776 mL | 0.472 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ezatiostat is a synthetic tripeptide analog of glutathione that inhibits the glutathione S-transferase P1-1 (GSTP1-1) [1].
Ezatiostat is a glutathione analog prodrug that has been shown to stimulate the proliferation of myeloid precursors. Ezatiostat is metabolized to TLK117 which shows selectivity to inhibit and bind to glutathione S-transferase P1-1 (GST P1-1). In addition, ezatiostat could activate the caspase-dependent pathway that help inhibit the emergence of malignant clones and may also contribute to apoptotic death and elimination by increasing reactive oxygen species (ROS) in dysplastic cells.
Ezatiostat has shown great stimulatory activity in vitro in human bone marrow progenitor cultures, as well as in several preclinical models of myelopoiesis in vivo. A multidose-escalation study of ezatiostat was performed for the treatment of myelodysplastic syndrome. Patients received 10 dose levels of ezatiostat tablets on days 1 to 7 of a 21-day cycle for a maximum of 8 cycles. Forty-five patients with low to intermediate-2 International Prognostic Scoring System risk myelodysplastic syndrome were enrolled. 17 hematologic improvement (HI) responses by International Working Group criteria were observed at dose range of 200 to 6000 mg/day , wherein 11 HI responses at dose range of 4000 to 6000 mg/day [1].
In randomized multicenter study of 2 extended dosing schedules of oral ezatiostat in low to intermediate-1 risk myelodysplastic syndrome. Patients were randomized by 1 stratification factor-baseline cytopenia to 1 of 2 extended dosing schedules. In general, 11 of 38 (29%) HI-Erythroid (HI-E) response was observed in patients who were red blood cell (RBC) transfusion-dependen. The median duration of HI-E response was 34 weeks. Ezatiostat is proven to cause clinically significant and sustained reduction in RBC transfusions, transfusion independence and multilineage responses in MDS patients [2].
References:
[1]. Raza A, Galili N, Smith S, et al. Phase 1 multicenter dose-escalation study of ezatiostat hydrochloride (TLK199 tablets), a novel glutathione analog prodrug, in patients with myelodysplastic syndrome. Blood, 2009, 113(26): 6533-6540.
[2]. Raza A, Galili N, Smith SE, et al. A phase 2 randomized multicenter study of 2 extended dosing schedules of oral ezatiostat in low to intermediate-1 risk myelodysplastic syndrome. Cancer, 2012, 118(8): 2138-2147.
- Conivaptan HCl
Catalog No.:BCC3756
CAS No.:168626-94-6
- TPEN
Catalog No.:BCC7913
CAS No.:16858-02-9
- AIDA
Catalog No.:BCC6841
CAS No.:168560-79-0
- H-Asp(OMe)-OH.HCl
Catalog No.:BCC2889
CAS No.:16856-13-6
- Fosbretabulin (Combretastatin A4 Phosphate (CA4P)) Disodium
Catalog No.:BCC4600
CAS No.:168555-66-6
- Dehydrogeijerin
Catalog No.:BCN7531
CAS No.:16850-91-2
- Z-D-Val-OH
Catalog No.:BCC2732
CAS No.:1685-33-2
- SHU 9119
Catalog No.:BCC6019
CAS No.:168482-23-3
- Epifriedelanol
Catalog No.:BCN1104
CAS No.:16844-71-6
- Rhodiocyanoside A
Catalog No.:BCN7852
CAS No.:168433-86-1
- Compound 401
Catalog No.:BCC7622
CAS No.:168425-64-7
- Tacrine hydrochloride
Catalog No.:BCC6869
CAS No.:1684-40-8
- H-Tyr-OtBu
Catalog No.:BCC3128
CAS No.:16874-12-7
- SGC707
Catalog No.:BCC6543
CAS No.:1687736-54-4
- Z-Tyr(tBu)-OH.DCHA
Catalog No.:BCC2745
CAS No.:16879-90-6
- RS 67333 hydrochloride
Catalog No.:BCC5693
CAS No.:168986-60-5
- RS 67506 hydrochloride
Catalog No.:BCC6878
CAS No.:168986-61-6
- Tropanyl phenylacetate
Catalog No.:BCN1927
CAS No.:1690-22-8
- 4'-O-Methylcoumestrol
Catalog No.:BCN7226
CAS No.:1690-62-6
- (2R,4R)-APDC
Catalog No.:BCC6969
CAS No.:169209-63-6
- MSPG
Catalog No.:BCC6819
CAS No.:169209-64-7
- MPPG
Catalog No.:BCC6818
CAS No.:169209-65-8
- MTPG
Catalog No.:BCC6820
CAS No.:169209-66-9
- Trametol
Catalog No.:BCN6924
CAS No.:169217-47-4
Phase 1 dose-ranging study of ezatiostat hydrochloride in combination with lenalidomide in patients with non-deletion (5q) low to intermediate-1 risk myelodysplastic syndrome (MDS).[Pubmed:22546242]
J Hematol Oncol. 2012 Apr 30;5:18.
BACKGROUND: Ezatiostat, a glutathione S-transferase P1-1 inhibitor, promotes the maturation of hematopoietic progenitors and induces apoptosis in cancer cells. RESULTS: Ezatiostat was administered to 19 patients with non-deletion(5q) myelodysplastic syndrome (MDS) at one of two doses (2000 mg or 2500 mg/day) in combination with 10 mg of lenalidomide on days 1-21 of a 28-day cycle. No unexpected toxicities occurred and the incidence and severity of adverse events (AEs) were consistent with that expected for each drug alone. The most common non-hematologic AEs related to Ezatiostat in combination with lenalidomide were mostly grade 1 and 2 fatigue, anorexia, nausea, diarrhea, and vomiting; hematologic AEs due to lenalidomide were thrombocytopenia, neutropenia, and anemia. One of 4 evaluable patients (25%) in the 2500/10 mg dose group experienced an erythroid hematologic improvement (HI-E) response by 2006 MDS International Working Group (IWG) criteria. Four of 10 evaluable patients (40%) in the 2000 mg/10 mg dose group experienced an HI-E response. Three of 7 (43%) red blood cell (RBC) transfusion-dependent patients became RBC transfusion independent, including one patient for whom prior lenalidomide monotherapy was ineffective. Three of 5 (60%) thrombocytopenic patients had an HI-platelet (HI-P) response. Bilineage HI-E and HI-P responses occurred in 3 of 5 (60%), 1 of 3 with HI-E and HI-N (33%), and 1 of 3 with HI-N and HI-P (33%). One of 3 patients (33%) with pancytopenia experienced a complete trilineage response. All multilineage responses were observed in the 2000/10 mg doses recommended for future studies. CONCLUSIONS: The tolerability and activity profile of Ezatiostat co-administered with lenalidomide supports the further development of Ezatiostat in combination with lenalidomide in MDS and also encourages studies of this combination in other hematologic malignancies where lenalidomide is active.
Ezatiostat hydrochloride for the treatment of myelodysplastic syndromes.[Pubmed:25724698]
Expert Opin Investig Drugs. 2015 May;24(5):725-33.
INTRODUCTION: Myelodysplastic syndromes (MDSs) are associated with significant morbidity due to ineffective hematopoiesis. Given the limited number of drugs approved by the FDA, there is a need for new therapeutic options. Ezatiostat is a novel agent targeting oxidative stress via inhibition of glutathione S-transferase 1. AREAS COVERED: Herein, the authors summarize the standard of care in order to build the framework for therapeutic advancements. The purpose of this paper is to review the body of preclinical and clinical research literature on the investigational agent Ezatiostat hydrochloride (TLK199) for the treatment of MDSs. The article includes details of the pathophysiology, pharmacology, toxicity and efficacy of Ezatiostat hydrochloride from controlled studies in patients with myelodysplasia. EXPERT OPINION: MDS clonal heterogeneity and clonal architecture complexity has presented a significant technical challenge in developing effective therapies. Ezatiostat offers a unique and specific mechanism to improve the transfusion burden associated with myelodysplasia. Since it is tolerable as a monotherapy, combining Ezatiostat with agents such as lenalidomide may have the most potential benefit.
Oral ezatiostat HCl (Telintra(R), TLK199) and idiopathic chronic neutropenia (ICN): a case report of complete response of a patient with G-CSF resistant ICN following treatment with ezatiostat, a glutathione S-transferase P1-1 (GSTP1-1) inhibitor.[Pubmed:22047626]
J Hematol Oncol. 2011 Nov 2;4:43.
Idiopathic chronic neutropenia (ICN) describes a heterogeneous group of hematologic diseases characterized by low circulating neutrophil levels often associated with recurrent fevers, chronic mucosal inflammation, and severe systemic infections. The severity and risk of complications, including serious infections, are inversely proportional to the absolute neutrophil count (ANC), with the greatest problems occurring in patients with an ANC of less than 0.5 x 109/L. This case report describes a 64-year-old female with longstanding rheumatoid arthritis who subsequently developed ICN with frequent episodes of sepsis requiring hospitalization and prolonged courses of antibiotics over a 4-year period. She was treated with granulocyte colony stimulating factors (G-CSF) but had a delayed, highly variable, and volatile response. She was enrolled in a clinical trial evaluating the oral investigational agent Ezatiostat. Ezatiostat, a glutathione S-transferase P1-1 inhibitor, activates Jun kinase, promoting the growth and maturation of hematopoietic progenitor stem cells. She responded by the end of the first month of treatment with stabilization of her ANC (despite tapering and then stopping G-CSF), clearing of fever, and healing of areas of infection. This ANC response to Ezatiostat treatment has now been sustained for over 8 months and continues. These results suggest potential roles for Ezatiostat in the treatment of patients with ICN who are not responsive to G-CSF, as an oral therapy alternative, or as an adjunct to G-CSF, and further studies are warranted.
Prediction of response to therapy with ezatiostat in lower risk myelodysplastic syndrome.[Pubmed:22559819]
J Hematol Oncol. 2012 May 6;5:20.
BACKGROUND: Approximately 70% of all patients with myelodysplastic syndrome (MDS) present with lower-risk disease. Some of these patients will initially respond to treatment with growth factors to improve anemia but will eventually cease to respond, while others will be resistant to growth factor therapy. Eventually, all lower-risk MDS patients require multiple transfusions and long-term therapy. While some patients may respond briefly to hypomethylating agents or lenalidomide, the majority will not, and new therapeutic options are needed for these lower-risk patients. Our previous clinical trials with Ezatiostat (Ezatiostat hydrochloride, Telentra(R), TLK199), a glutathione S-transferase P1-1 inhibitor in clinical development for the treatment of low- to intermediate-risk MDS, have shown significant clinical activity, including multilineage responses as well as durable red-blood-cell transfusion independence. It would be of significant clinical benefit to be able to identify patients most likely to respond to Ezatiostat before therapy is initiated. We have previously shown that by using gene expression profiling and grouping by response, it is possible to construct a predictive score that indicates the likelihood that patients without deletion 5q will respond to lenalidomide. The success of that study was based in part on the fact that the profile for response was linked to the biology of the disease. METHODS: RNA was available on 30 patients enrolled in the trial and analyzed for gene expression on the Illumina HT12v4 whole genome array according to the manufacturer's protocol. Gene marker analysis was performed. The selection of genes associated with the responders (R) vs. non-responders (NR) phenotype was obtained using a normalized and rescaled mutual information score (NMI). CONCLUSIONS: We have shown that an Ezatiostat response profile contains two miRNAs that regulate expression of genes known to be implicated in MDS disease pathology. Remarkably, pathway analysis of the response profile revealed that the genes comprising the jun-N-terminal kinase/c-Jun molecular pathway, which is known to be activated by Ezatiostat, are under-expressed in patients who respond and over-expressed in patients who were non-responders to the drug, suggesting that both the biology of the disease and the molecular mechanism of action of the drug are positively correlated.


