Cyanidin ChlorideCAS# 528-58-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 528-58-5 | SDF | Download SDF |
| PubChem ID | 68247 | Appearance | Red-black powder |
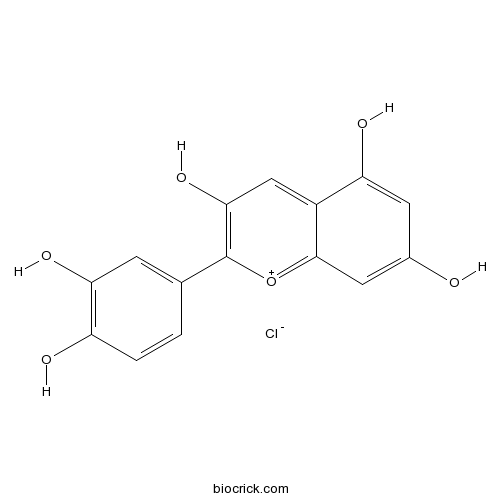
| Formula | C15H11O6Cl | M.Wt | 322.70 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Cyanidol chloride; 3,3',4',5,7-Pentahydroxyflavylium chloride | ||
| Solubility | Soluble in methan | ||
| Chemical Name | 2-(3,4-dihydroxyphenyl)chromenylium-3,5,7-triol;chloride | ||
| SMILES | C1=CC(=C(C=C1C2=C(C=C3C(=CC(=CC3=[O+]2)O)O)O)O)O.[Cl-] | ||
| Standard InChIKey | COAWNPJQKJEHPG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H10O6.ClH/c16-8-4-11(18)9-6-13(20)15(21-14(9)5-8)7-1-2-10(17)12(19)3-7;/h1-6H,(H4-,16,17,18,19,20);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cyanidin Chloride, the main phenolic antioxidant in the grape (Vitis vinifera), in particular in the liposomal forms, could be used for treatment of diabetes mellitus complications. It has a dual effect on RANKL-induced osteoclastogenesis, exhibits therapeutic potential in prevention of osteoclasts related bone disorders. |
| Targets | HbA1c glycation | LXR-β | NFATc1 | c-Fos | Mitf |
| In vitro | Inhibition of lipid peroxidation and the active oxygen radical scavenging effect of anthocyanin pigments isolated from Phaseolus vulgaris L.[Pubmed: 8831722]Biochem Pharmacol. 1996 Oct 11;52(7):1033-9.
|
| In vivo | Treatment of diabetes in the mouse model by delphinidin and cyanidin hydrochloride in free and liposomal forms.[Pubmed: 24108435]Planta Med. 2013 Nov;79(17):1599-604.Cyanidin Chloride and delphinidin are the main phenolic antioxidants in the grape (Vitis vinifera). The aim of this study was to investigate the in vitro and in vivo inhibitory effects of delphinidin and Cyanidin Chloride in the free and liposomal forms on the albumin glycation reaction.
|
| Cell Research | Dual Effect of Cyanidin on RANKL-Induced Differentiation and Fusion of Osteoclasts.[Pubmed: 25545964]J Cell Physiol. 2014 Dec 24.Bone homeostasis is maintained by the balance between osteoblastic bone formation and osteoclastic bone resorption. Osteoclasts are multinucleated cells derived from hematopoietic stem cells (HSCs) or monocyte/macrophage progenitor cells and formed by osteoclasts precursors (OCPs) fusion. Cyanidin Chloride is an anthocyanin widely distributed in food diet with novel antioxidant activity. However, the effect of Cyanidin Chloride on osteoclasts is still unknown.
|

Cyanidin Chloride Dilution Calculator

Cyanidin Chloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0989 mL | 15.4943 mL | 30.9885 mL | 61.9771 mL | 77.4713 mL |
| 5 mM | 0.6198 mL | 3.0989 mL | 6.1977 mL | 12.3954 mL | 15.4943 mL |
| 10 mM | 0.3099 mL | 1.5494 mL | 3.0989 mL | 6.1977 mL | 7.7471 mL |
| 50 mM | 0.062 mL | 0.3099 mL | 0.6198 mL | 1.2395 mL | 1.5494 mL |
| 100 mM | 0.031 mL | 0.1549 mL | 0.3099 mL | 0.6198 mL | 0.7747 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Delphinidin chloride
Catalog No.:BCN3015
CAS No.:528-53-0
- Fisetin
Catalog No.:BCN5024
CAS No.:528-48-3
- Magnolol
Catalog No.:BCN5687
CAS No.:528-43-8
- H-Phe(3,4-DiCl)-OH
Catalog No.:BCC3178
CAS No.:52794-99-7
- H-D-Phe(3,4-DiCl)-OH
Catalog No.:BCC3179
CAS No.:52794-98-6
- PHA 568487
Catalog No.:BCC7574
CAS No.:527680-57-5
- Medicarpin 3-O-glucoside
Catalog No.:BCN7773
CAS No.:52766-70-8
- beta-Asarone
Catalog No.:BCN5685
CAS No.:5273-86-9
- Isoelemicin
Catalog No.:BCN4760
CAS No.:5273-85-8
- Scillascillin
Catalog No.:BCN5684
CAS No.:52706-07-7
- Ginsenoside Rd
Catalog No.:BCN1074
CAS No.:52705-93-8
- Herbacetin
Catalog No.:BCN1268
CAS No.:527-95-7
- (±)-Galgravin
Catalog No.:BCN8283
CAS No.:528-63-2
- Isobavachromene
Catalog No.:BCN3192
CAS No.:52801-22-6
- L-Quisqualic acid
Catalog No.:BCC6568
CAS No.:52809-07-1
- Dalbergiphenol
Catalog No.:BCN7451
CAS No.:52811-31-1
- Cearoin
Catalog No.:BCN7772
CAS No.:52811-37-7
- Olean-12-ene-3,11-diol
Catalog No.:BCN5686
CAS No.:5282-14-4
- 20-Hydroxyecdysone
Catalog No.:BCN5688
CAS No.:5289-74-7
- Chamazulene
Catalog No.:BCC8145
CAS No.:529-05-5
- Ombuin
Catalog No.:BCN5691
CAS No.:529-40-8
- Myricetin
Catalog No.:BCN5692
CAS No.:529-44-2
- Gentisein
Catalog No.:BCN3356
CAS No.:529-49-7
- Azaleatin
Catalog No.:BCN8207
CAS No.:529-51-1
Dual Effect of Cyanidin on RANKL-Induced Differentiation and Fusion of Osteoclasts.[Pubmed:25545964]
J Cell Physiol. 2016 Mar;231(3):558-67.
Bone homeostasis is maintained by the balance between osteoblastic bone formation and osteoclastic bone resorption. Osteoclasts are multinucleated cells derived from hematopoietic stem cells (HSCs) or monocyte/macrophage progenitor cells and formed by osteoclasts precursors (OCPs) fusion. Cyanidin is an anthocyanin widely distributed in food diet with novel antioxidant activity. However, the effect of cyanidin on osteoclasts is still unknown. We investigated the effect of cyanidin on RANKL-induced osteoclasts differentiation and cell fusion. The results showed that cyanidin had a dual effect on RANKL-induced osteoclastogenesis. Lower dosage of cyanidin (< 1 microg/ml) has a promoting effect on osteoclastogenesis while higher dosage of cyanidin (> 10 microg/ml) has an inhibitory effect. Fusogenic genes like CD9, ATP6v0d2, DC-STAMP, OC-STAMP, and osteoclasts related genes like NFATc1, mitf, and c-fos were all regulated by cyanidin consistent to its dual effect. Further exploration showed that low concentration of cyanidin could increase osteoclasts fusion whereas higher dosage of cyanidin lead to the increase of LXR-beta expression and activation which is suppressive to osteoclasts differentiaton. All these results showed that cyanidin exhibits therapeutic potential in prevention of osteoclasts related bone disorders.
Inhibition of lipid peroxidation and the active oxygen radical scavenging effect of anthocyanin pigments isolated from Phaseolus vulgaris L.[Pubmed:8831722]
Biochem Pharmacol. 1996 Oct 11;52(7):1033-9.
No attention has been paid to anthocyanin pigments from the viewpoint of inhibitors of lipid peroxidation and scavengers of active oxygen radicals; therefore, we investigated the antioxidative, radical scavenging, and inhibitory effects on lipid peroxidation by UV light irradiation of three anthocyanin pigments, pelargonidin 3-O-beta-D-glucoside (P3G), cyanidin 3-O-beta-D-glucoside (C3G), and delphinidin 3-O-beta-D-glucoside (D3G), isolated from the Phaseolus vulgaris L. seed coat, and their aglycons, pelargonidin chloride (Pel), Cyanidin Chloride (Cy), and delphinidin chloride (Del). All pigments had strong antioxidative activity in a liposomal system and reduced the formation of malondialdehyde by UVB irradiation. On the other hand, the extent of antioxidative activity in a rat liver microsomal system and the scavenging effect of hydroxyl radicals (-OH) and superoxide anion radicals (O2-) were influenced by their own structures.
Treatment of diabetes in the mouse model by delphinidin and cyanidin hydrochloride in free and liposomal forms.[Pubmed:24108435]
Planta Med. 2013 Nov;79(17):1599-604.
Cyanidin and delphinidin are the main phenolic antioxidants in the grape (Vitis vinifera). The aim of this study was to investigate the in vitro and in vivo inhibitory effects of delphinidin and Cyanidin Chloride in the free and liposomal forms on the albumin glycation reaction. Delphinidin and Cyanidin Chlorides were encapsulated in the liposomes using an extrusion method. The rate of albumin glycation was evaluated using the ELISA method. Finally, in vivo anti-glycation of delphinidin and Cyanidin Chloride in the free and liposomal forms in diabetic mice was investigated. The encapsulation efficacies of delphinidin and Cyanidin Chloride in the liposomes were 89.05 % +/- 0.18 and 85.00 % +/- 0.15, respectively. In vitro treatment with 100 mg/mL delphinidin and Cyanidin Chloride in free forms could reduce the rate of albumin glycation to 30.50 +/- 3.46 and 46.00 +/- 2.50 %, respectively. Under identical conditions, the delphinidin and Cyanidin Chloride-loaded liposomes could reduce the rate of albumin glycation to 8.50 +/- 2.10 and 14.60 +/- 3.60 %, respectively. In vivo testing showed that anti-glycation activity of delphinidin and cyanidin in loaded forms was higher than in free forms. The daily administration of 100 mg/kg delphinidin chloride-loaded liposomes to diabetic mice at eight weeks could decrease the rate of albumin and HbA1c glycation to 46.35 +/- 1.20 and 3.60 +/- 0.25 %, respectively. Moreover, under identical conditions, the loaded liposomes with Cyanidin Chloride could decrease the rate of albumin and HbA1c glycation to 55.56 +/- 1.32 and 4.95 +/- 0.20 %, respectively. The findings showed that delphinidin and Cyanidin Chloride, in particular in the liposomal forms, could be used for treatment of diabetes mellitus complications.


