AzaleatinCAS# 529-51-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 529-51-1 | SDF | Download SDF |
| PubChem ID | 5281604 | Appearance | Yellow powder |
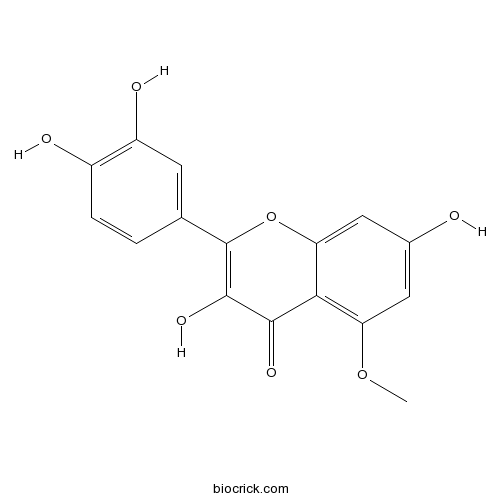
| Formula | C16H12O7 | M.Wt | 316.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-(3,4-dihydroxyphenyl)-3,7-dihydroxy-5-methoxychromen-4-one | ||
| SMILES | COC1=C2C(=CC(=C1)O)OC(=C(C2=O)O)C3=CC(=C(C=C3)O)O | ||
| Standard InChIKey | RJBAXROZAXAEEM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H12O7/c1-22-11-5-8(17)6-12-13(11)14(20)15(21)16(23-12)7-2-3-9(18)10(19)4-7/h2-6,17-19,21H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Azaleatin is a natural product Rhododendron simsii. |
| In vitro | Flavonoid patterns and phytogeography: the genus Rhododendron section Vireya.[Reference: WebLink]Phytochemistry, 1986, 25(7):1641-1643.
|

Azaleatin Dilution Calculator

Azaleatin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1616 mL | 15.8078 mL | 31.6156 mL | 63.2311 mL | 79.0389 mL |
| 5 mM | 0.6323 mL | 3.1616 mL | 6.3231 mL | 12.6462 mL | 15.8078 mL |
| 10 mM | 0.3162 mL | 1.5808 mL | 3.1616 mL | 6.3231 mL | 7.9039 mL |
| 50 mM | 0.0632 mL | 0.3162 mL | 0.6323 mL | 1.2646 mL | 1.5808 mL |
| 100 mM | 0.0316 mL | 0.1581 mL | 0.3162 mL | 0.6323 mL | 0.7904 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Gentisein
Catalog No.:BCN3356
CAS No.:529-49-7
- Myricetin
Catalog No.:BCN5692
CAS No.:529-44-2
- Ombuin
Catalog No.:BCN5691
CAS No.:529-40-8
- Chamazulene
Catalog No.:BCC8145
CAS No.:529-05-5
- 20-Hydroxyecdysone
Catalog No.:BCN5688
CAS No.:5289-74-7
- Olean-12-ene-3,11-diol
Catalog No.:BCN5686
CAS No.:5282-14-4
- Cearoin
Catalog No.:BCN7772
CAS No.:52811-37-7
- Dalbergiphenol
Catalog No.:BCN7451
CAS No.:52811-31-1
- L-Quisqualic acid
Catalog No.:BCC6568
CAS No.:52809-07-1
- Isobavachromene
Catalog No.:BCN3192
CAS No.:52801-22-6
- (±)-Galgravin
Catalog No.:BCN8283
CAS No.:528-63-2
- Cyanidin Chloride
Catalog No.:BCN1231
CAS No.:528-58-5
- Scutellarein
Catalog No.:BCN5380
CAS No.:529-53-3
- Prunin
Catalog No.:BCN5693
CAS No.:529-55-5
- Genistin
Catalog No.:BCN2396
CAS No.:529-59-9
- Euxanthone
Catalog No.:BCN5694
CAS No.:529-61-3
- 6,7-Dihydroxy-4-Methylcoumarin
Catalog No.:BCC8287
CAS No.:529-84-0
- Motilin (human, porcine)
Catalog No.:BCC5894
CAS No.:52906-92-0
- PRIMA-1MET
Catalog No.:BCC2414
CAS No.:5291-32-7
- Dammaradienyl acetate
Catalog No.:BCN5689
CAS No.:52914-31-5
- Neosophoramine
Catalog No.:BCN5690
CAS No.:52932-74-8
- Nanaomycin A
Catalog No.:BCC3611
CAS No.:52934-83-5
- N-(2,6-Diphenylmethyl)-1-piperazine acetylamine
Catalog No.:BCC9052
CAS No.:5294-61-1
- Genz-644282
Catalog No.:BCC1592
CAS No.:529488-28-6
Anti-hyperuricemic effect of isorhamnetin in cultured hepatocytes and model mice: structure-activity relationships of methylquercetins as inhibitors of uric acid production.[Pubmed:30603920]
Cytotechnology. 2019 Feb;71(1):181-192.
Hyperuricemia is an important risk factor for gout. Isorhamnetin (3'-O-methylquercetin) is an O-methylated flavonol, which occurs in onion, almond and sea buckthorn. It is also one of the metabolites of quercetin in mammals. In the present study, we investigated anti-hyperuricemic effect of isorhamnetin adopting both cultured hepatocytes and mice with hyperuricemia induced by purine bodies. In cultured hepatocytes, isorhamnetin as well as quercetin significantly and dose-dependently inhibited uric acid (UA) production. We also examined the inhibitory effects on UA production of other mono-methylquercetins, i.e., tamarixetin, 3-O-methylquercetin, Azaleatin, and rhamnetin in addition to isorhamnetin for studying their structure-activity relationships. From the results obtained, hydroxyl groups at C-3, C-5, and especially C-7, but not C-3' and C-4' of quercetin are demonstrated to play a critical role in suppressing UA production in the AML12 hepatocytes. Oral administration of isorhamnetin significantly reduced plasma and hepatic UA levels in the hyperuricemic model mice. Isorhamnetin also decreased hepatic xanthine oxidase (XO) activity without changes in XO protein expression, indicating that anti-hyperuricemic effect of isorhamnetin could be, at least partly, attributable to suppression of UA production by directly inhibiting XO activity in the liver. These findings demonstrate that isorhamnetin has a potent anti-hyperuricemic effect and may be a potential candidate for prevention and remediation of hyperuricemia.
Isolation and characterization of free radical scavenging flavonoid glycosides from the flowers of Spartium junceum by activity-guided fractionation.[Pubmed:11091001]
J Ethnopharmacol. 2000 Dec;73(3):471-8.
Spartium junceum L. (Fabaceae) flowers are used for the treatment of peptic ulcers in Turkish folk medicine. The possible superoxide dismutase-like activity of the extracts, fractions and constituents obtained through activity-guided fractionation were studied by using in vitro electron spin resonance spectrometry, in order to explain the role of antioxidant principles in the potent antiulcerogenic activity of the extract. Despite the fact that the triterpene, spartitrioside, which was previously reported as the active antiulcerogenic constituent of the flowers was found almost inactive, the flavonoid-rich fractions showed potent antioxidant activity. Five flavonoid glycosides bearing catechol structure in ring B were isolated from the butanol extract and their structures were elucidated using 1H- and 13C-NMR techniques as isoquercitrin (quercetin 3beta-glucoside) (1,); luteolin 4'beta-glucoside (2); quercetin 3, 4'-diglucoside (3); Azaleatin 3beta-glucoside (quercetin 5-methylether 3beta-glucoside) (4), quercetin 4'beta-glucoside (5). Flavonoids (2) and (4) showed the highest in vitro antioxidant activity with 22.59 and 19.08 U/ml, respectively.


