Genz-644282Non-camptothecin inhibitor of topoisomerase I CAS# 529488-28-6 |
- Gatifloxacin
Catalog No.:BCC1064
CAS No.:112811-59-3
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 529488-28-6 | SDF | Download SDF |
| PubChem ID | 10294813 | Appearance | Powder |
| Formula | C22H21N3O5 | M.Wt | 407.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
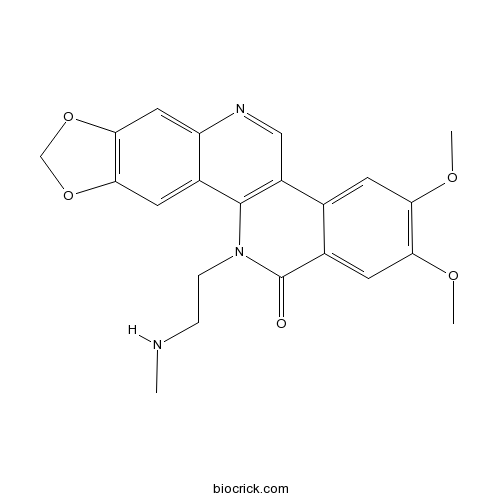
| SMILES | CNCCN1C2=C(C=NC3=CC4=C(C=C32)OCO4)C5=CC(=C(C=C5C1=O)OC)OC | ||
| Standard InChIKey | BAORCAMWLWRZQG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H21N3O5/c1-23-4-5-25-21-14-8-19-20(30-11-29-19)9-16(14)24-10-15(21)12-6-17(27-2)18(28-3)7-13(12)22(25)26/h6-10,23H,4-5,11H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Genz-644282 is a non-camptothecin topoisomerase I inhibitor, used for cancer research.In Vitro:Genz-644282 is a topoisomerase I inhibitor. Genz-644282 shows potent activities against 29 human tumor cell lines with IC50s ranging from 1.8 nM to 1.8 μM[1]. Genz-644282 suppresses the PPTP cell lines, with IC50s of 0.2-21.9 nM, and the mean IC50 value is 1.2 nM[2]. Genz-644282 is potent at trapping Top1-DNA covalent cleavage complexes. Genz-644282 (0.1 μM) induces γH2AX foci in human colon cancer HCT116 cells and breast cancer MCF7 cells. Genz-644282 is cytotoxic on the CPT-resistant human cancer cell lines[3].In Vivo:Genz-644282 (1-4 mg/kg) is active when administered intravenously to the mice. Genz-644282 (2.7 mg/kg, i.v.) causes tumor growth delay (TGD) of 34 days in the human HCT-116 colon cancer xenograft, 27 days in the human HT-29 colon carcinoma xenograft and mice bearing the NCI-H460 human non-small cell lung carcinoma. Genz-644282 (2 mg/kg, i.v.) results in a TGD of 33 days in the human HCT-15 colon carcinoma xenograft, and 28 days in mice bearing LOX-IMVI melanoma. Moreover, Genz-644282 (1 mg/kg, i.v.) leads to 14 days of TGD in mice bearing the DLD-1 human colon carcinoma xenograft. Genz-644282 (1.7 mg/kg, i.v.) also produces a TGD of 23 days in mice bearing 786-O tumors and 33 days in NCI-H1299 human non-small cell lung carcinoma xenograft[1]. Genz644282 at maximum tolerated dose (MTD, 4 mg/kg) results in maintained complete responses (MCR) in 6/6 evaluable solid tumor models. Genz644282 (2 mg/kg) induces CR or MCR in 3/3 tumor models and causes objective regressions in 7 of 17 (41%) models, but there are no objective responses at 1 mg/kg[2]. References: | |||||

Genz-644282 Dilution Calculator

Genz-644282 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4545 mL | 12.2723 mL | 24.5447 mL | 49.0894 mL | 61.3617 mL |
| 5 mM | 0.4909 mL | 2.4545 mL | 4.9089 mL | 9.8179 mL | 12.2723 mL |
| 10 mM | 0.2454 mL | 1.2272 mL | 2.4545 mL | 4.9089 mL | 6.1362 mL |
| 50 mM | 0.0491 mL | 0.2454 mL | 0.4909 mL | 0.9818 mL | 1.2272 mL |
| 100 mM | 0.0245 mL | 0.1227 mL | 0.2454 mL | 0.4909 mL | 0.6136 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: 1.2 nM [1] Genz-644282 [8,9-dimethoxy-5-(2-N-methylaminoethyl)-2,3-methylenedioxy-5H-dibenzo[c,h][1,6]naphthyridin-6-one] has emerged as a promising candidate of non-Camptothecin topoisomerase I inhibitor for antitumor agents. in vitro: Genz-644282 demonstrated potent cytotoxic activity with a median IC(50) of 1.2?nM (range 0.2-21.9?nM) [1]. Limited cross-resistance to Genz-644282 was also found in the Top1 knockdown colon cancer (HCT116) and breast cancer (MCF7) cell lines and in human adenocarcinoma cells (KB31/KBV1) that overexpress (P-glycoprotein, ABCB1), a member of the ATP-binding cassette family of cell surface transport proteins known to confer MDR [3]. in vivo: Genz-644282 at its MTD (4?mg/kg) induced maintained complete responses (MCR) in 6/6 evaluable solid tumor models. At 2?mg/kg Genz-644282 induced CR or MCR in 3/3 tumor models relatively insensitive to topotecan, but there were no objective responses at 1?mg/kg [1]. Genz-644282 was tolerated at doses up to 4 mg/kg when administered intravenously on alternate days, and the compound was active at doses from 1 to 4 mg/kg. The efficacy of Genz-644282 was compared with irinotecan in 4 human colon carcinoma xenograft models. In the human HCT-116 colon cancer xenograft, TGD values were 14 days for irinotecan (60 mg/kg) and 34 days for Genz-644282 (2.7 mg/kg), giving maximum test to control ratios (T/Cs) of 23.7% and 16.8%, respectively [2]. Clinical trial: Dose-Escalation Study to Assess the Safety and Tolerability of Genz-644282 in Patients With Solid Tumors. Phase 1
- N-(2,6-Diphenylmethyl)-1-piperazine acetylamine
Catalog No.:BCC9052
CAS No.:5294-61-1
- Nanaomycin A
Catalog No.:BCC3611
CAS No.:52934-83-5
- Neosophoramine
Catalog No.:BCN5690
CAS No.:52932-74-8
- Dammaradienyl acetate
Catalog No.:BCN5689
CAS No.:52914-31-5
- PRIMA-1MET
Catalog No.:BCC2414
CAS No.:5291-32-7
- Motilin (human, porcine)
Catalog No.:BCC5894
CAS No.:52906-92-0
- 6,7-Dihydroxy-4-Methylcoumarin
Catalog No.:BCC8287
CAS No.:529-84-0
- Euxanthone
Catalog No.:BCN5694
CAS No.:529-61-3
- Genistin
Catalog No.:BCN2396
CAS No.:529-59-9
- Prunin
Catalog No.:BCN5693
CAS No.:529-55-5
- Scutellarein
Catalog No.:BCN5380
CAS No.:529-53-3
- Azaleatin
Catalog No.:BCN8207
CAS No.:529-51-1
- Ajugol
Catalog No.:BCN2883
CAS No.:52949-83-4
- Acetylisocupressic acid
Catalog No.:BCN5695
CAS No.:52992-82-2
- Prednisone
Catalog No.:BCC4957
CAS No.:53-03-2
- Estrone
Catalog No.:BCN2201
CAS No.:53-16-7
- Mitotane (Lsodren)
Catalog No.:BCC3815
CAS No.:53-19-0
- Cocaine hydrochloride
Catalog No.:BCC5943
CAS No.:53-21-4
- Methylprednisolone acetate
Catalog No.:BCC9043
CAS No.:53-36-1
- Oxandrolone
Catalog No.:BCC5242
CAS No.:53-39-4
- Dehydroepiandrosterone
Catalog No.:BCN2202
CAS No.:53-43-0
- Indomethacin
Catalog No.:BCC3794
CAS No.:53-86-1
- L-Picein
Catalog No.:BCC8336
CAS No.:530-14-3
- Deoxyvasicinone
Catalog No.:BCN5697
CAS No.:530-53-0
Genz-644282, a novel non-camptothecin topoisomerase I inhibitor for cancer treatment.[Pubmed:21415217]
Clin Cancer Res. 2011 May 1;17(9):2777-87.
PURPOSE: Genz-644282 [8,9-dimethoxy-5-(2-N-methylaminoethyl)-2,3-methylenedioxy-5H-dibenzo[c,h][1,6]na phthyridin-6-one] has emerged as a promising candidate for antitumor agents. This report describes the bone marrow colony-forming unit, granulocyte macrophage (CFU-GM) and tumor cell CFU activity of topoisomerase I (Top1) inhibitors, such as Genz-644282, topotecan, irinotecan/SN-38, and ARC-111, and examines their activity in several human tumor xenograft models. EXPERIMENTAL DESIGN: Colony-forming assays were conducted with mouse and human bone marrow and eight human tumor cell lines. In addition, 29 human tumor cell lines representing a range of histology and potential resistance mechanisms were assayed for sensitivity to Genz-644282 in a 72-hour exposure assay. The efficacy of Genz-644282 was compared with standard anticancer drugs (i.e., irinotecan, docetaxel, and dacarbazine) in human tumor xenografts of colon cancer, renal cell carcinoma, non-small cell lung cancer, and melanoma. RESULTS: Human bone marrow CFU-GM was more sensitive to the Top1 inhibitors than was mouse bone marrow CFU-GM. The ratio of mouse to human IC(90) values was more than 10 for the camptothecins and less than 10 for Genz-644282, which had more potency as a cytotoxic agent toward human tumor cells in culture than the camptothecins in the colony-forming and 72-hour proliferation assays. Genz-644282 has superior or equal antitumor activity in the human tumor xenografts than the standard drug comparators. CONCLUSIONS: On the basis of preclinical activity and safety, Genz-644282 was selected for development and is currently undergoing phase 1 clinical trial.
Molecular and cellular pharmacology of the novel noncamptothecin topoisomerase I inhibitor Genz-644282.[Pubmed:21636699]
Mol Cancer Ther. 2011 Aug;10(8):1490-9.
Camptothecin derivatives are powerful anticancer drugs because of their ability to trap topoisomerase I (Top1)-DNA cleavage complexes. However, they exhibit clinical limitations due to the instability of their alpha-hydroxylactone six-membered E-ring structure. In addition, they exhibit bone marrow and intestinal toxicity, especially in adults, and are drug efflux substrates. Here, we report a novel Top1 inhibitor, Genz-644282. We show that Genz-644282 and its metabolites induce Top1 cleavage at similar, as well as unique genomic positions, compared with camptothecin. The compound also induces protein-linked DNA breaks and Top1-DNA cleavage complexes that persist longer after compound removal than camptothecin. Concentration-dependent and persistent gammaH2AX formation was readily observed in cells treated with Genz-644282, and was present in greater than 50% of the cell population following 24 hours compound exposure. The compound shows partial cross-resistance in cell lines resistant to camptothecin. These cell lines include the human prostate DU145RC0.1 and the leukemic CEM/C2 cells. Limited cross-resistance to Genz-644282 was also found in the Top1 knockdown colon cancer (HCT116) and breast cancer (MCF7) cell lines and in human adenocarcinoma cells (KB31/KBV1) that overexpress (P-glycoprotein, ABCB1), a member of the ATP-binding cassette family of cell surface transport proteins known to confer MDR. Together, our results provide the first molecular and cellular characterization of Genz-644282 and its clinically relevant metabolites.
Testing of the topoisomerase 1 inhibitor Genz-644282 by the pediatric preclinical testing program.[Pubmed:21548007]
Pediatr Blood Cancer. 2012 Feb;58(2):200-9.
BACKGROUND: Genz-644282 is a novel non-camptothecin topoisomerase I poison that is in clinical development. PROCEDURES: Genz-644282 was tested against the PPTP in vitro panel (0.1 nM to 1 microM), and in vivo using three times per week x 2 schedule repeated at day 21 at its maximum tolerated dose (MTD) of 4 mg/kg. Subsequently Genz-644282 was tested at 4, 3, 2, and 1 mg/kg in 3 models to assess the dose-response relationship. mRNA gene signatures predictive for Genz-644282 response in vitro were applied to select 15 tumor models that were evaluated prospectively. RESULTS: In vitro, Genz-644282 demonstrated potent cytotoxic activity with a median IC(50) of 1.2 nM (range 0.2-21.9 nM). In vivo, Genz-644282 at its MTD (4 mg/kg) induced maintained complete responses (MCR) in 6/6 evaluable solid tumor models. At 2 mg/kg Genz-644282 induced CR or MCR in 3/3 tumor models relatively insensitive to topotecan, but there were no objective responses at 1 mg/kg. Further testing at 2 mg/kg showed that Genz-644282 induced objective regressions in 7 of 17 (41%) models. There was a significant correlation between predictive response scores based on Affymetrix U133Plus2 baseline tumor expression profiles and the observed in vivo responses to Genz-644282. CONCLUSIONS: Genz-644282 was highly active within a narrow dose range (2-4 mg/kg), typical of other topoisomerase I poisons. As with other topoisomerase I poisons, how accurately these data will translate to clinical activity will depend upon the drug exposures that can be achieved in children treated with this agent.


