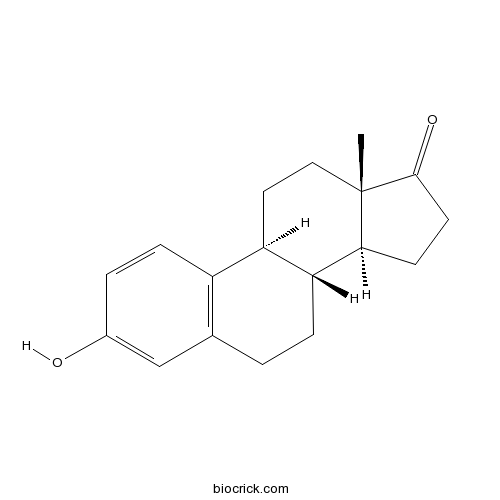
EstroneEstrogenic hormone CAS# 53-16-7 |
- Fulvestrant
Catalog No.:BCC1081
CAS No.:129453-61-8
- (Z)-2-decenoic acid
Catalog No.:BCC1295
CAS No.:15790-91-7
- Bazedoxifene
Catalog No.:BCC1411
CAS No.:198481-32-2
- Bazedoxifene acetate
Catalog No.:BCC1412
CAS No.:198481-33-3
- (E)-2-Decenoic acid
Catalog No.:BCC1292
CAS No.:334-49-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 53-16-7 | SDF | Download SDF |
| PubChem ID | 5870 | Appearance | Powder |
| Formula | C18H22O2 | M.Wt | 270.37 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | DMSO : 33.33 mg/mL (123.28 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | (8R,9S,13S,14S)-3-hydroxy-13-methyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-one | ||
| SMILES | CC12CCC3C(C1CCC2=O)CCC4=C3C=CC(=C4)O | ||
| Standard InChIKey | DNXHEGUUPJUMQT-CBZIJGRNSA-N | ||
| Standard InChI | InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Estrone,a steroid known to play an important role as precursor of 17 beta-estradiol,especially in postmenopausal women, it inhibits the BCRP-mediated drug efflux and overcome drug resistance.The widely distributed estrone esters in food and their relatively high concentrations may result in high free hormone intakes in humans, the continued and massive intake of estrone may enhance tissue deposition and lead to obesity. |
| Targets | Estrogen receptor | Progestogen receptor |
| In vitro | The effect of particle size on sorption of estrogens, androgens and progestagens in aquatic sediment.[Pubmed: 26094244]J Hazard Mater. 2015 May 29;299:112-121.There is growing concern about the biologic effects of steroid hormones in impacted waterways. There is increasing evidence of enhanced transport and biological effects stemming from steroid hormones associated with soils or sediments; however, there are limited studies evaluating how steroid hormone distribution between various particle sizes within whole sediments affects steroid fate.
Estrone and 17beta-estradiol reverse breast cancer resistance protein-mediated multidrug resistance.[Pubmed: 11927002]Jpn J Cancer Res. 2002 Mar;93(3):231-5.Breast cancer resistance protein (BCRP), an adenosine triphosphate-binding cassette transporter, confers resistance to a series of anticancer reagents, including mitoxantrone, SN-38 and topotecan.
|
| In vivo | Estrone in food: a factor influencing the development of obesity?[Pubmed: 10654162]Eur J Nutr. 1999 Oct;38(5):247-53.Estrone is a relatively abundant hormone widely distributed in tissues of animal and plant origin. It is a mild estrogen that induces increases in body weight in experimental animals. The relative abundance of Estrone esters in animal tissues suggests that it may also be found in foods, from which it may alter the mechanisms of body weight control.
To measure the total Estrone content in food and to determine whether this may affect body weight.
|
| Animal Research | Inhibitory effect of a steroidal antiestrogen (EM-170) on estrone-stimulated growth of 7,12-dimethylbenz(a)anthracene (DMBA)-induced mammary carcinoma in the rat.[Pubmed: 7749151]Breast Cancer Res Treat. 1995 Mar;33(3):237-44.Recently, compounds having pure antiestrogenic activity have become available.
|

Estrone Dilution Calculator

Estrone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6986 mL | 18.4932 mL | 36.9864 mL | 73.9727 mL | 92.4659 mL |
| 5 mM | 0.7397 mL | 3.6986 mL | 7.3973 mL | 14.7945 mL | 18.4932 mL |
| 10 mM | 0.3699 mL | 1.8493 mL | 3.6986 mL | 7.3973 mL | 9.2466 mL |
| 50 mM | 0.074 mL | 0.3699 mL | 0.7397 mL | 1.4795 mL | 1.8493 mL |
| 100 mM | 0.037 mL | 0.1849 mL | 0.3699 mL | 0.7397 mL | 0.9247 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Estrone (E1, and also oestrone) is an estrogenic hormone secreted by the ovary as well as adipose tissue with the chemical name of 3-hydroxyestra-1,3,5(10)-triene-17-one and the chemical formula C18H22O2. Estrone is an odorless, solid crystalline powder, white in color with a melting point of 254.5 °C and a specific gravity of 1.23. Estrone is one of several natural estrogens, which also include estriol and estradiol. Estrone is the least abundant of the three hormones; estradiol is present almost always in the reproductive female body, and estriol is abundant primarily during pregnancy.
- Prednisone
Catalog No.:BCC4957
CAS No.:53-03-2
- Acetylisocupressic acid
Catalog No.:BCN5695
CAS No.:52992-82-2
- Ajugol
Catalog No.:BCN2883
CAS No.:52949-83-4
- Genz-644282
Catalog No.:BCC1592
CAS No.:529488-28-6
- N-(2,6-Diphenylmethyl)-1-piperazine acetylamine
Catalog No.:BCC9052
CAS No.:5294-61-1
- Nanaomycin A
Catalog No.:BCC3611
CAS No.:52934-83-5
- Neosophoramine
Catalog No.:BCN5690
CAS No.:52932-74-8
- Dammaradienyl acetate
Catalog No.:BCN5689
CAS No.:52914-31-5
- PRIMA-1MET
Catalog No.:BCC2414
CAS No.:5291-32-7
- Motilin (human, porcine)
Catalog No.:BCC5894
CAS No.:52906-92-0
- 6,7-Dihydroxy-4-Methylcoumarin
Catalog No.:BCC8287
CAS No.:529-84-0
- Euxanthone
Catalog No.:BCN5694
CAS No.:529-61-3
- Mitotane (Lsodren)
Catalog No.:BCC3815
CAS No.:53-19-0
- Cocaine hydrochloride
Catalog No.:BCC5943
CAS No.:53-21-4
- Methylprednisolone acetate
Catalog No.:BCC9043
CAS No.:53-36-1
- Oxandrolone
Catalog No.:BCC5242
CAS No.:53-39-4
- Dehydroepiandrosterone
Catalog No.:BCN2202
CAS No.:53-43-0
- Indomethacin
Catalog No.:BCC3794
CAS No.:53-86-1
- L-Picein
Catalog No.:BCC8336
CAS No.:530-14-3
- Deoxyvasicinone
Catalog No.:BCN5697
CAS No.:530-53-0
- 2,6-Dimethoxy-1,4-benzoquinone
Catalog No.:BCN5698
CAS No.:530-55-2
- Syringic acid
Catalog No.:BCN5699
CAS No.:530-57-4
- Sinapic acid
Catalog No.:BCN3539
CAS No.:530-59-6
- CDI (1,1′-Carbonyldiimidazole)
Catalog No.:BCC2809
CAS No.:530-62-1
The effect of particle size on sorption of estrogens, androgens and progestagens in aquatic sediment.[Pubmed:26094244]
J Hazard Mater. 2015 Dec 15;299:112-21.
There is growing concern about the biologic effects of steroid hormones in impacted waterways. There is increasing evidence of enhanced transport and biological effects stemming from steroid hormones associated with soils or sediments; however, there are limited studies evaluating how steroid hormone distribution between various particle sizes within whole sediments affects steroid fate. In this study, sorption of 17beta-estradiol, Estrone, progesterone, and testosterone was evaluated to different size fractions of two natural sediments, a silty loam and a sandy sediment, to determine the steroid sorption capacity to each fraction and distribution within the whole sediment. Sorption isotherms for all steroid hormones fit linear sorption models. Sorption capacity was influenced more by organic carbon content than particle size. Interactions between size fractions were found to affect the distribution of steroids within the whole sediments. All four steroids preferentially sorbed to the clay and colloids in the silty loam sediment at the lowest aqueous concentration (1 ng/L) and as aqueous concentration increased, the distribution of sorbed steroid was similar to the distribution by weight of each size fraction within the whole sediment. In the sandy sediment, preferential sorption to fine particles was observed.
Inhibitory effect of a steroidal antiestrogen (EM-170) on estrone-stimulated growth of 7,12-dimethylbenz(a)anthracene (DMBA)-induced mammary carcinoma in the rat.[Pubmed:7749151]
Breast Cancer Res Treat. 1995 Mar;33(3):237-44.
Recently, compounds having pure antiestrogenic activity have become available. In this study, we examined the activity of the new steroidal antiestrogen EM-170 (N-n-butyl, N-methyl-11-(16' alpha-chloro-3',17' alpha-dihydroxy-estra-1',3',5'-(10')-trien-7' alpha-yl) undecanamide) on the growth of 7,12-dimethylbenz(a)anthracene (DMBA)-induced mammary carcinoma stimulated by treatment with Estrone (E1), a steroid known to play an important role as precursor of 17 beta-estradiol (E2), especially in postmenopausal women. Twenty-five days after ovariectomy (OVX), tumor volume in control OVX animals decreased to 51.4 +/- 11% of the initial volume; treatment with E1, administered by Silastic implants, stimulated tumor growth to 179 +/- 21%. Treatment with the antiestrogen EM-170 at a dose of 200 micrograms (twice daily) not only completely reversed the stimulatory effect of E1, but also inhibited tumor growth to 30.5 +/- 9.6%, an effect that is 41% (P < 0.01 vs OVX control) greater than that of ovariectomy alone. At a relatively low dose of 40 micrograms (twice daily), 20 days of treatment with EM-170 reversed by 55% the stimulatory effect of E1 (1.0 micrograms, subcutaneously, twice daily) on tumor growth in OVX animals. On the other hand, the antiestrogen also induced a significant inhibitory effect on 17 beta-hydroxysteroid dehydrogenase (17 beta-HSD) activity in the DMBA-induced mammary tumors, an effect that is in agreement with the marked reduction caused by the same treatment on tumor estradiol (E2) levels in E1-treated OVX animals.(ABSTRACT TRUNCATED AT 250 WORDS)
Estrone and 17beta-estradiol reverse breast cancer resistance protein-mediated multidrug resistance.[Pubmed:11927002]
Jpn J Cancer Res. 2002 Mar;93(3):231-5.
Breast cancer resistance protein (BCRP), an adenosine triphosphate-binding cassette transporter, confers resistance to a series of anticancer reagents, including mitoxantrone, SN-38 and topotecan. In the present study, we found that Estrone and 17beta-estradiol potentiated the cytotoxicity of mitoxantrone, SN-38 and topotecan in BCRP-transduced K562 cells (K562 / BCRP). These estrogens showed only a marginal effect, or none, in parental K562 cells. Estrone and 17beta-estradiol increased the cellular accumulation of topotecan in K562 / BCRP cells, but not in K562 cells, suggesting that these estrogens inhibit the BCRP-mediated drug efflux and overcome drug resistance.


