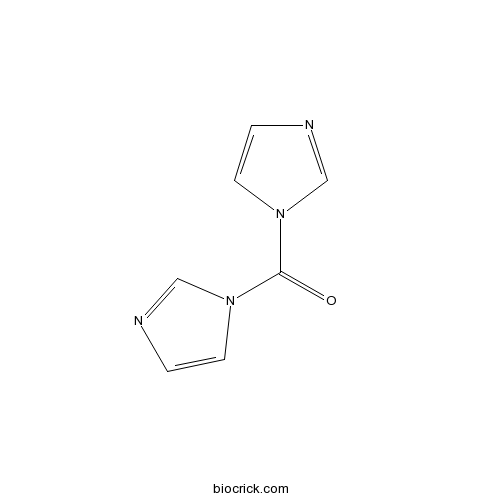
CDI (1,1′-Carbonyldiimidazole)Coupling agent for synthesis of dipolar polyamides CAS# 530-62-1 |
- LY2606368
Catalog No.:BCC4105
CAS No.:1234015-52-1
- CHIR-124
Catalog No.:BCC3750
CAS No.:405168-58-3
- AZD7762
Catalog No.:BCC2555
CAS No.:860352-01-8
- MK-8776 (SCH-900776)
Catalog No.:BCC3817
CAS No.:891494-63-6
- LY2603618
Catalog No.:BCC3923
CAS No.:911222-45-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 530-62-1 | SDF | Download SDF |
| PubChem ID | 68263 | Appearance | Powder |
| Formula | C7H6N4O | M.Wt | 162.15 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in water or 1% acetic acid | ||
| Chemical Name | di(imidazol-1-yl)methanone | ||
| SMILES | C1=CN(C=N1)C(=O)N2C=CN=C2 | ||
| Standard InChIKey | PFKFTWBEEFSNDU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C7H6N4O/c12-7(10-3-1-8-5-10)11-4-2-9-6-11/h1-6H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

CDI (1,1′-Carbonyldiimidazole) Dilution Calculator

CDI (1,1′-Carbonyldiimidazole) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.1671 mL | 30.8356 mL | 61.6713 mL | 123.3426 mL | 154.1782 mL |
| 5 mM | 1.2334 mL | 6.1671 mL | 12.3343 mL | 24.6685 mL | 30.8356 mL |
| 10 mM | 0.6167 mL | 3.0836 mL | 6.1671 mL | 12.3343 mL | 15.4178 mL |
| 50 mM | 0.1233 mL | 0.6167 mL | 1.2334 mL | 2.4669 mL | 3.0836 mL |
| 100 mM | 0.0617 mL | 0.3084 mL | 0.6167 mL | 1.2334 mL | 1.5418 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: Not available.
The common method of peptide synthesis is not feasible for industry use due to its poor yields. CDI, a new peptide forming reagent, is applied recently as a more direct agent for the formation of acyl-imidazoles. [1]
In vitro: CDI can be prepared straightforwardly by the reaction of phosgene with four equivalents of imidazole under anhydrous conditions. It served as a convenient reagent for peptide synthesis since its side products, carbon dioxide and imidazole, were safe. Moreover, the carbon dioxide evolution was suggested to provide driving force for the peptide synthesis. A typical peptide-formation reaction was started by dissolving 0.010 mole acylamino acid in 10 ml tetrahydrofuran (THF), and after this, the evolution of carbon dioxide could be immediately observed. An hour later, the required amino acid or peptide ester was added with an amount of 0.010 molar. The reaction would be last for 15 min, but longer standing is probably beneficial. The formed peptide was then purified by wiping off the solvent under vacuum followed by washing the residue with acid, saturated bicarbonate and finally water.[1]
In vivo: So far, no in vivo data has been reported.
Clinical trial: So far, no clinical trial has been conducted.
Reference:
[1] Paul R, Anderson GW. N,N'-Carbonyldiimidazole, a new peptide forming reagent. J. Am. Chem. Soc. 1960 Sep; 82: 4597-600.
- Sinapic acid
Catalog No.:BCN3539
CAS No.:530-59-6
- Syringic acid
Catalog No.:BCN5699
CAS No.:530-57-4
- 2,6-Dimethoxy-1,4-benzoquinone
Catalog No.:BCN5698
CAS No.:530-55-2
- Deoxyvasicinone
Catalog No.:BCN5697
CAS No.:530-53-0
- L-Picein
Catalog No.:BCC8336
CAS No.:530-14-3
- Indomethacin
Catalog No.:BCC3794
CAS No.:53-86-1
- Dehydroepiandrosterone
Catalog No.:BCN2202
CAS No.:53-43-0
- Oxandrolone
Catalog No.:BCC5242
CAS No.:53-39-4
- Methylprednisolone acetate
Catalog No.:BCC9043
CAS No.:53-36-1
- Cocaine hydrochloride
Catalog No.:BCC5943
CAS No.:53-21-4
- Mitotane (Lsodren)
Catalog No.:BCC3815
CAS No.:53-19-0
- Estrone
Catalog No.:BCN2201
CAS No.:53-16-7
- Salinomycin
Catalog No.:BCC1916
CAS No.:53003-10-4
- Murralongin
Catalog No.:BCN5696
CAS No.:53011-72-6
- T-5224
Catalog No.:BCC5383
CAS No.:530141-72-1
- Scutebarbatine J
Catalog No.:BCN8134
CAS No.:960302-85-6
- Morellic acid
Catalog No.:BCN3073
CAS No.:5304-71-2
- 9,13-Epidioxy-8(14)-abieten-18-oic acid
Catalog No.:BCN1426
CAS No.:5309-35-3
- Dichotomin
Catalog No.:BCN2836
CAS No.:53093-47-3
- Androsin
Catalog No.:BCN3842
CAS No.:531-28-2
- Coniferin
Catalog No.:BCN5700
CAS No.:531-29-3
- Scopolin
Catalog No.:BCN5701
CAS No.:531-44-2
- 7-Methoxycoumarin
Catalog No.:BCN2707
CAS No.:531-59-9
- Esculin
Catalog No.:BCN5904
CAS No.:531-75-9
1,1'-Carbonyldiimidazole (CDI) Mediated Coupling and Cyclization To Generate [1,2,4]Triazolo[4,3-a]pyridines.[Pubmed:26808327]
Org Lett. 2016 Feb 5;18(3):560-3.
An operationally efficient CDI mediated tandem coupling and cyclization reaction to generate [1,2,4]triazolo[4,3-a]pyridines has been reported. The reaction conditions and scope were investigated, and the methodology was demonstrated in batch mode as well as in a continuous process.


